
Ernest Rutherford developed Rutherford's atomic model . Ernest Rutherford (1871–1937) developed the rules of radioactive decay, found alpha and beta rays, and hypothesized the nuclear structure of the atom. In 1908, he was awarded the Chemistry Nobel Prize.
Rutherford, a master experimenter, made many discoveries in radioactivity and nuclear physics. He established the rules of radioactive decay, recognized alpha particles as helium nuclei, and found alpha and beta rays. Most importantly, he proposed a model for the atom's nuclear structure. Rutherford's lab studies revealed that when alpha particles are fired into gas atoms, some violently deflect, suggesting that the bulk of the atomic mass is located in a dense, positively charged center area.Also Check - Electrophiles Formula
Introduction To Atom
Before understanding Rutherford’s atomic model, one must know the atom and its importance. An atom is the smallest substance with all an element's chemical characteristics. A gold coin, for instance, is just a massive number of gold atoms that have been molded into the shape of a coin together with very minute amounts of other contaminating elements. Gold atoms can never be reduced to a smaller size and preserve their characteristics. The little subatomic particles that make up a gold atom give it its qualities.Also Check - Nucleophile Formula
There are two zones in an atom. The first is the minuscule atomic nucleus, located in the middle of the atom and includes positively charged protons and neutral, uncharged neutrons.Properties Of Matter
Matter is everything that occupies space and has mass; in other words, matter is the "stuff" that makes up the cosmos. The basic constituents of all stuff are called elements. They don't change into other elements through normal chemical processes and have distinct chemical and physical properties.Also Check - Discovery of Proton Formula
For example, both carbon and gold are elements. Only 92 out of the 118 elements are found naturally. The compounds that are left are unstable and were only produced in laboratories.Early Life Of Rutherford
The fourth of 12 children, Rutherford was raised on a farm in New Zealand. After graduating from the University of New Zealand, he worked with rowdy students. He was freed from this obligation by receiving a scholarship to Cambridge University and enrolled there as J. J. Thomson's first graduate student at the Cavendish Laboratory. He started working with radio transmission there, joined Thomson's ongoing study of electrical conduction via gases, and then switched to the topic of radioactivity that Henri Becquerel and Pierre and Marie Curie had just discovered.Rutherford’s Atom Model Experiment
In an experiment, Rutherford bombarded a thin sheet of gold with alpha particles and then tracked the paths the particles took after they made contact with the foil. In his experiment, Rutherford fired high-energy beams of alpha particles at a 100 nm-thick gold sheet from a radioactive source. He surrounded the thin gold foil with a fluorescent zinc sulfide screen to analyze the deflection the alpha particles experienced. Rutherford made certain claims that were at odds with Thomson's atomic model.Also Check - Modern Periodic Table Formula
Rutherford’s Atom Model
Rutherford suggested the atomic structure of elements based on his observations and deductions. The Rutherford atomic model states:- A little volume contains both the mass and positive charge of an atom. He referred to the atom's nucleus.
- According to Rutherford's hypothesis, a negatively charged electron shell surrounds an atom's nucleus. In addition, he claimed that the electrons that orbit the nucleus move in a circular pattern at very high speeds.
- He coined the word orbit to describe their elliptical paths.
-
The mass of positively charged particles that make up the nucleus is held together by a strong electrostatic force of attraction that attracts negatively charged and positively charged electrons.
[caption id="attachment_17335" align="aligncenter" width="300"]
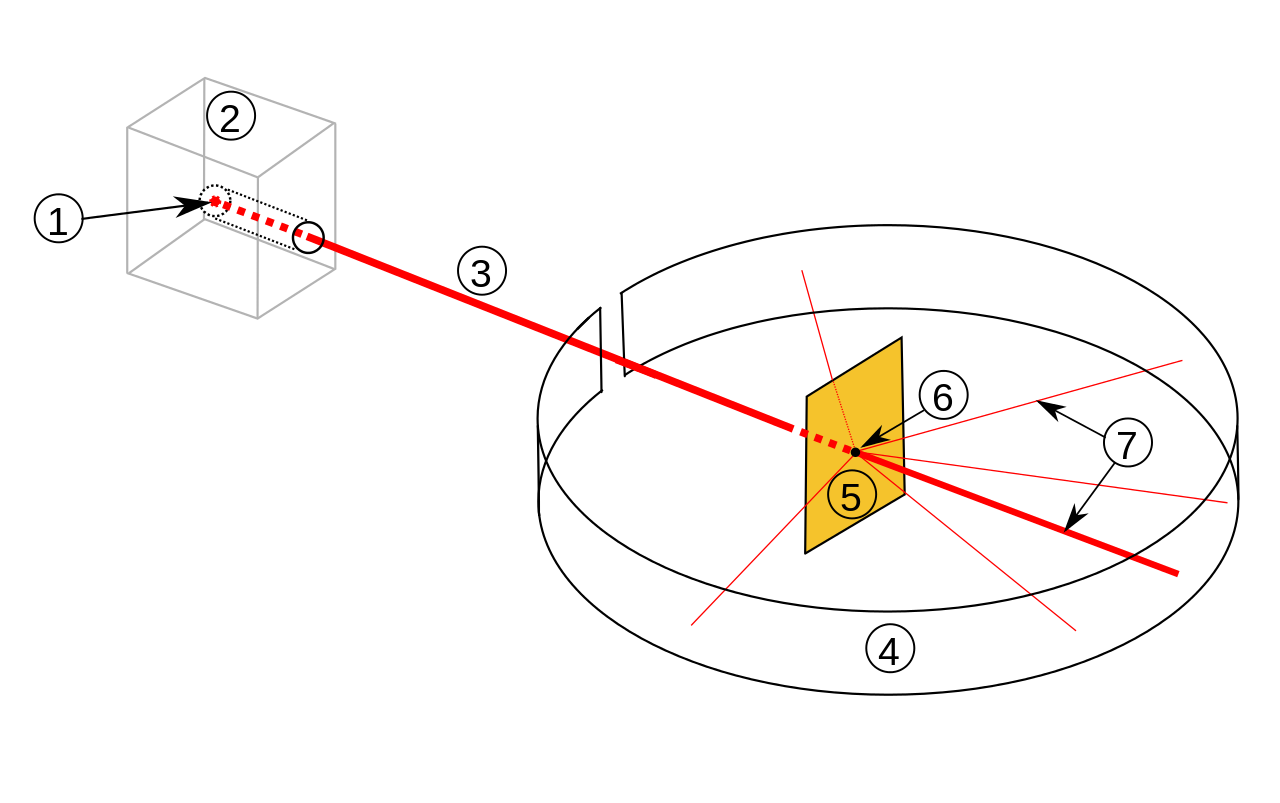 Rutherford's atom model experiment[/caption]
Rutherford's atom model experiment[/caption]
Rutherford’s Observations
Rutherford's observations led him to draw the following conclusions:- Most of the space in an atom is unoccupied since a significant portion of the -particles blasted toward the gold sheet went through the sheet without deflection.
- Because the gold sheet somewhat deflected some of the alpha particles, the positive charge in an atom is not spread evenly. An atom's positive charge is confined to a relatively tiny volume.
- Only a handful of the alpha particles had deflection angles that were almost 180 degrees, meaning that very few of the alpha particles were deflected back. As a result, the positively charged particles' volume is extremely tiny about the total volume of an atom.
Also Check - Periodic Acid Formula
Rutherford’s Atom Model Limitations
According to Rutherford, the electrons go along predetermined routes known as orbits as they circle the nucleus. According to Maxwell, an electron rotating around the nucleus should create electromagnetic radiation because accelerating charged particles release such radiation. This radiation would transport energy from the electron's speed at the expense of smaller orbits. In the nucleus, the electrons would eventually collapse. The speed of the electron would be used to carry energy through this radiation at the price of smaller orbits. The electrons would finally collapse in the nucleus. Calculations show that an electron would enter the nucleus under the Rutherford model in less than 10-8 seconds. The Rutherford model, which did not fit with Maxwell's theory, could not explain the stability of an atom. Since the Rutherford model mentioned nothing about how the electrons were ordered in an atom, it was an incomplete theory, one of its flaws.Rutherford's Atomic Model FormulaFAQs
What made Rutherford's atomic model unique?
The first person to recognize an atom's nucleus was Rutherford. He struck gold with alpha particles and discovered positively charged material inside the atom.
What is explained by Rutherford's model?
The Rutherford atomic model states that an atom's positive charge and mass are contained in a very tiny volume.
How did Rutherford discover the atomic model?
The gold foil experiment is Ernest Rutherford's most well-known experiment. A piece of gold foil was the target of an alpha particle beam. While some alpha particles were dispersed backward, most went through the foil.
Rutherford used gold foil for what purpose?
Rutherford desired a metal sheet that could be as thin as feasible for the scattering experiment. Out of all known metals, gold is the most malleable. It is simple to transform into fragile sheets.
Who contradicted Rutherford's theory?
Having joined Rutherford in 1912, Bohr realized that Rutherford's model wasn't exactly correct. It should be exceedingly unstable based on the classical physics laws.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









