

We will discuss in this article the chloroacetic acid formula, also known as monochloroacetic acid formula or chloroethanoic acid formula. Chloroacetic acid is an organochlorine compound that is an effective building block. It is known as 2-chloroacetic acid, 2-chloroethanoic acid, and is a molecular or chemical formula of C2H3ClO2.
A colourless to light-brown crystal in its solid form sinks and dissolves in water. In its aqueous form, it appears as a colourless solution. It is widely used as an herbicide, bacteriostat, and preservative.What is Chloroacetic Acid?
An inorganic acid called chloroacetic acid is widely used in gold refining. Additionally, it is a chlorocarboxylic acid which is then an acetic acid with a 2-chloro substituent. It is also a conjugate acid of chloroacetate, a light brown, crystalline substance that sinks in water and is also soluble in it. Since it is transported as a molten solid, it can cause thermal burns.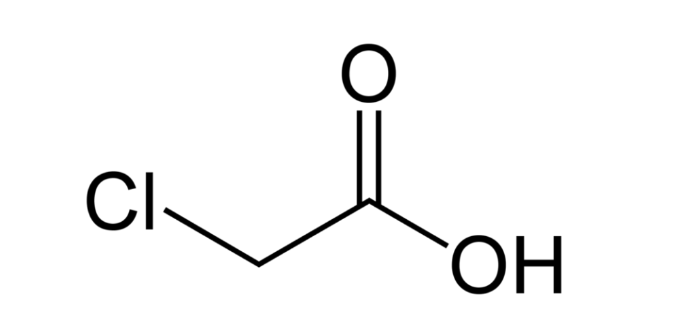 Moreover, chloroacetic acid is a colorless solution of crystalline solids that are white in color. Furthermore, the concentration of this acid can be up to 80%. In addition to being toxic, chloroacetic acid is also corrosive to metals and tissues. One can use it as a preservative and a bacteriostat, also known as monochloroacetic acid in the industrial sector.
Moreover, chloroacetic acid is a colorless solution of crystalline solids that are white in color. Furthermore, the concentration of this acid can be up to 80%. In addition to being toxic, chloroacetic acid is also corrosive to metals and tissues. One can use it as a preservative and a bacteriostat, also known as monochloroacetic acid in the industrial sector.
Also Read : Malic Acid Formula
Derivation of Chloroacetic Acid Formula
A chloroacetic acid's formula is C2H3O2Cl. Monocloroacetic acid can be prepared industrially in two months. Additionally, acetic anhydride is used as a catalyst to chlorinate acetic acid. CH3CO2H+Cl2→ClCH2CO2H+HCl Chloroacetic acid can also be obtained by hydrolyzing trichloroethylene with a catalyst, such as sulfuric acid. CCl2=CHCl+2H2O→ClCH2CO2H+2HClAlso Check - Calcium Bromide Formula
Properties of Chloroacetic Acid
A solid chloroacetic acid is a light-brown, crystalline substance that is colorless and soluble in water. Acetic acid is also explosive by nature, corrosive to metals, and hygroscopic. In addition, chloroacetic acid has a pungent odor that resembles strong vinegar. The crystals of chloroacetic acid are hygroscopic. At 189.3 °C, chloroacetic acid reaches its boiling point, while it melts at 63.0 °C. Additionally, the flashpoint of this acid is 126 °C. Its solubility surpasses 100 mg/mL at 68° F and it can dissolve in diethyl ether, methanol, acetone, ethanol, benzene, and chloroform. However, carbon tetrachloride, chlorinated hydrocarbons, and hydrocarbons only slightly dissolve this acid.Also Check - Caffeine Chemical Formula
The chloroacetic acid (solid) density is 1.58 at 20 °C/20 °C. The vapor density of this acid is 6.5X10-2 mm Hg (8.68X10-3 kPa) at 25 °C. A fire can cause hazardous decomposition products to form. Hydrogen chloride gas and carbon oxides can be produced during this decomposition.| Chemical formula | C2H3ClO2 |
| Molecular weight | 94.49 g/mol |
| Density | 1.58 g/cm3 |
| Melting point | 63 °C |
| Boiling point | 189.3 °C |
Uses of Chloroacetic Acid
The thickeners carboxymethyl cellulose and carboxymethyl starch are prepared with chloroacetic acid in its largest-scale application. There are many reactions that take advantage of the high reactivity of the C–Cl bond, which is why chloroacetic acid is so widely used in the manufacture of pesticides, drugs, and dyes. In addition to glyphosate, chloroacetic acid is the precursor to MCPA (2-methyl-4-chlorophenoxyacetic acid). In addition, dimethoate is prepared through alkylation with chloroacetic acid, which is converted to chloroacetyl chloride. Furthermore, chloroacetyl chloride is a precursor to adrenaline (epinephrine). In addition to being used as a stabilizer in PVC, thioglycolic acid is also used as a component in cosmetics. Displacement of chloride by sulfur leads to the formation of thioglycolic acid. Benzofuran is produced by O-alkylation of salicylaldehyde with chloroacetic acid, followed by decarboxylation of the ether formed.Health Hazards
When 2-chloroacetic acid comes into contact with the skin or eyes, it causes irritation and burning sensation, and when inhaled it severely damages mucous membranes. This material is very toxic. The lethal dose is 50-500 mg/kg of body weight. Swallowing can lead to peritonitis, as well as depression of the central nervous system and respiratory system.C<span style=
What is Chloroacetic Acid (CAA)?
Chloroacetic Acid (CAA) is a chemical compound with the formula CH2ClCOOH. It is a strong organic acid and a colorless, pungent-smelling liquid that is widely used in the chemical industry for various applications.
What are the common uses of Chloroacetic Acid?
Chloroacetic Acid is used in the production of various chemicals, including herbicides, dyes, and pharmaceuticals. It is also employed as a reagent in organic synthesis and as a pH regulator in industrial processes.
Is Chloroacetic Acid hazardous to health and the environment?
Yes, Chloroacetic Acid is corrosive and can cause severe skin, eye, and respiratory irritation. It is also toxic to aquatic life and poses environmental risks, so its handling and disposal must adhere to strict safety and environmental guidelines.
How can one safely handle Chloroacetic Acid?
Safe handling of Chloroacetic Acid requires the use of appropriate personal protective equipment, such as gloves and eye protection. It should be handled in a well-ventilated area, and spills should be promptly and safely cleaned up.
Can Chloroacetic Acid be neutralized or diluted for disposal?
Yes, Chloroacetic Acid can be neutralized with a suitable base, like sodium hydroxide, to form a less hazardous compound. However, this neutralization process must be carried out with caution and in accordance with local regulations before disposal.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.










