
Calcium Bromide is formed by one calcium atom and two bromine atoms. This article explains the Calcium Bromide formula, also called Calcium Dibromide formula or Calcium Bromatum formula.
Anhydrous is a hygroscopic colorless crystal with a sharp saline taste and is soluble in water and alcohol. It reacts with calcium carbonate (CaCO3) and calcium oxide with hydrobromic acid (HBr). It can also be produced by reacting calcium metal with elemental bromine. Drilling fluids commonly use it as a dense aqueous solution. Furthermore, it has applications such as neuroses medication, food preservatives, freezing mixtures, fire retardants, and photography.
Calcium (Ca) is indispensable for all living creatures, including humans. It is the most abundant mineral in the body and essential for good health. We need to eat a certain amount of calcium to build and maintain strong bones and maintain a sound correspondence between the mind and different body parts.
Among the halogens on the periodic table, bromine is known by its chemical name br. The bromine substance Br2 is a rosy-colored fluid. It is never normally found in its basic structure but inorganic mixtures, referred to as bromides, and regular organo-bromine compounds. Some of these can be found in soil, salt, and air.\
Also Read: Acetamide Formula
What is Calcium Bromide?
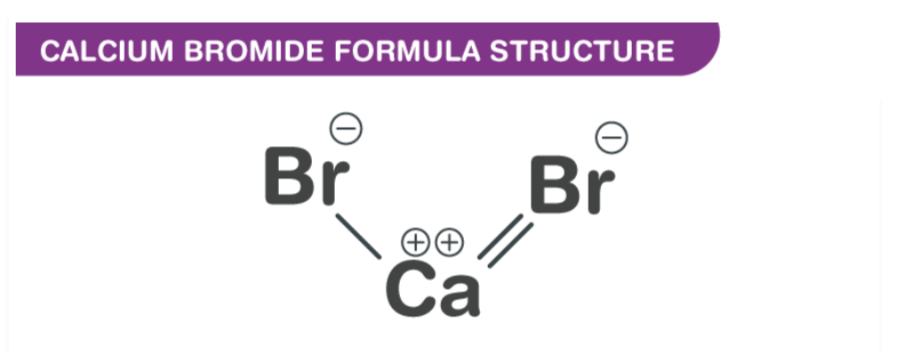
The molecular formula of Calcium Bromide is CaBr2. It is composed of one calcium atom and two bromine atoms.
Anhydrous, hygroscopic, colorless crystals with a pungent salty taste are anhydrous, hygroscopic, and soluble in water and alcohol. Calcium carbonate (CaCO3) and calcium oxide react with hydrobromic acid (HBr), or calcium metal with elemental bromine can be used to make it. As well as drilling fluids, it is widely used as a food preservative, frozen mixes, flame retardant, and photography agent.
Calcium Bromide Formula Structure
Calcium Bromide is a hydrated salt with two atoms of water but can also be found as a hexahydrate salt. Its chemical formula is CaBr2. It comprises one cation, Ca2+, and two anions, Br–. An octahedron of Ca focuses is bound to a group of six anion Br– by ionic bonds.
Properties Of Calcium Bromide Formula
- Upon dissolving in water, calcium bromide forms a colorless solution.
- In addition to being highly soluble in water, calcium bromide can also be dissolved in acetone and alcohol.
- Hygroscopic means calcium bromide can absorb moisture from the air and dissolve in it.
- In water, calcium bromide dissociates into calcium cations and bromide anions, and it can also react with other ions to form various bromides.
- When heated with air, calcium bromide reacts with oxygen to form calcium oxide and bromine.
Also Check - Ascorbic Acid Formula
| Chemical formula | CaBr2 |
| Molecular weight | 199.89 g/mol (anhydrous) 235.98 g/mol (dihydrate) |
| Density | 3.353 g/cm3 |
| Boiling point | 1,815 °C (anhydrous) 810 °C (dihydrate) |
| Melting point | 730 °C |
Calcium Bromide Formula Preparation
Alternatively, we can prepare calcium bromide by reacting calcium oxide with hydrobromic acid or by reacting metallic calcium with elemental bromine:
CaO+2HBr→CaBr2+H2O
Ca+Br2→CaBr2
Applications Of Calcium Bromide Formula
Also, it has essential applications in neuroses medication, food preservatives, freezing mixtures, fire retardants, and photography.
Food industries use this salt as a preservative, a component of freezing mixtures, a fire retardant, a wood preservative, and a dehydrating agent.
We spray calcium bromide on coal before combustion to control upstream oil and gas mercury emissions. Calcium bromide helps control wellbore pressures during completion and workover operations. During combustion, calcium bromide reacts with elemental mercury to produce ionic mercury species, which can then be captured downstream by pollution control equipment.
Calcium Bromide Formula FAQs
What is the chemical formula for Calcium Bromide?
What is the molar mass of Calcium Bromide (CaBr2)?
Is Calcium Bromide soluble in water?
Is Calcium Bromide toxic or hazardous?










