
Sodium nitride formula is Na 3 N. It is an inorganic compound with High instability among alkali metal nitrides. It is formed by depositing sodium and nitrogen atomic beams onto a low-temperature sapphire substrate. It rapidly decomposes into its constituent elements.
Sodium Nitride Formula: Sodium, with the chemical symbol Na, possesses an electron configuration of 1s 2 2s 2 2p 6 3s 1 . It is a metallic element, bearing a positive charge of +1. In contrast, Nitrogen, symbolized as N, has an electron configuration of 1s 2 2s 2 2p 3 , characterizing it as a nonmetal with a negative charge of -3. To balance these charges, we can express the compound Sodium Nitride as Na 3 N. Notably, Sodium Nitride lacks carbon-hydrogen bonds, making it inherently unstable and prone to decompose into sodium and nitrogen ions.
Sodium nitride formula is Na 3 N. Sodium Nitride is an inorganic compound. It consists of sodium (Na) and nitrogen (N). Sodium has an atomic number of 11 with electron configuration of 1s 2 2s 2 2p 6 4s 1 , while nitrogen has an atomic number of 7 with an electron configuration of 1s 2 2s 2 2p 3
This inorganic chemical, Sodium Nitride, exhibits exceptional instability and is synthesized by combining atomic constituents, depositing sodium under low-temperature conditions. It assumes the form of a white crystalline solid with a density of 2.26g/mL. Its practical applications include serving as a salt substitute and a food preservative. Sodium nitride formula is Na 3 N. They represents an ionic compound resulting from interactions between metals and nonmetals, which is characteristic of all ionic substances.
Sodium Nitride Formula
Sodium nitride formula is Na 3 N. It is an inorganic compound that can be synthesized by amalgamating sodium and nitrogen on a sapphire substrate at low temperatures.
Sodium Nitride comprises sodium (Na), characterized by the symbol Na, an atomic number of 11, and an electron configuration of 1s 2 2s 2 2p 6 4s1. Nitrogen (N), symbolized as N, is a chemical element with an atomic number of 7 and an electron configuration of 1s 2 2s 2 2p 3 .
This inorganic substance, Sodium Nitride, exhibits a remarkable degree of instability. Its production involves the combination of atomic components and the deposition of sodium at reduced temperatures. It manifests as a white crystalline solid with a density of 2.26g/mL. Sodium Nitride finds application as a salt substitute and a food preservative.
Sodium Nitride Formula Structure
Sodium nitride formula is Na 3 N. The structure of Sodium Nitride consists of a central nitrogen atom bonded to three sodium atoms. Sodium Nitride, an inorganic compound with the chemical formula Na 3 N, is depicted in the structure below:
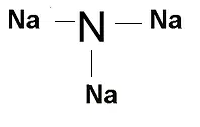
The formation of this structure for Na 3 N is a result of its ionic nature, involving the interaction of a metal and a non-metal. In ionic compounds, valence electrons are transferred from the metal to the non-metal. In the case of Na 3 N, three sodium ions are formed, followed by nitrogen. Sodium, located in Group I of the periodic table, hold one valence electron, resulting in a +1 charge.
Nitrogen, belonging to Group 15 on the periodic table, has five valence electrons, leading to a 3- charge. The attraction between the positive and negative charges culminates in the creation of an ionic bond.
Sodium Nitride Preparation
Sodium nitride formula is Na 3 N. To create Sodium Nitride, two distinct methods can be used. One approach involves the decomposition of Sodium Amide, resulting in the production of Sodium Nitride and hydrogen:
3 NaNH 2 + Heat → Na 3 N + H 2
Alternatively, Sodium Nitride can be prepared by reacting sodium with plasma-activated nitrogen on a metal surface, as demonstrated in the equation below:
2 N 2 + 3 Na → Na 3 N + 3/2 N 2
Sodium Nitride Formula Properties
Chemical Formula: Sodium Nitride exhibits various properties:
Sodium nitride formula is Na 3 N.Molecular Weight: Sodium Nitride has a molecular weight of 82.98 g/mol.
Density: It possesses a density of 2.17 g/cm3.
Melting Point: The compound does not have a boiling point, but it does have a melting point of 87°C.
Appearance: It appears as a reddish-brown or dark blue solid.
Solubility: It is soluble in water.
Stability: Sodium Nitride is unstable and rapidly decomposes into its constituent elements, following the equation:
2Na 3 N → 6 Na + N 2 .
Reaction with Hydrochloric Acid: When Sodium Nitride reacts with hydrochloric acid, it yields sodium chloride and ammonia:
Na 3 N + 3 HCl → 3 NaCl + NH 3 .
Reaction with Water: In the presence of water, Sodium Nitride reacts to produce sodium hydroxide and ammonia:
Na 3 N + 3 H 2 O → 3 NaOH + NH 3 .
Harmful Effects of Sodium Nitride
The Harmful effects of Sodium Nitride include:
- Irritation of the eyes and nose.
- The potential to induce gastroenteritis and abdominal pains.
- Skin irritation, along with eye irritation that can result in symptoms like redness, itching, and pain.
- Nausea, vomiting, rapid heartbeat, irregular breathing, and, in severe cases, the possibility of coma and even fatality if significant exposure occurs.
- Repeated exposure may lead to headaches and mental impairment.
Uses of Sodium Nitride
Sodium Nitride finds various applications, including:
- Serving as a food preservative and a salt substitute.
- Functioning as a microbial agent in the preservation of meat and fish.
- Facilitating the preservation of large quantities of various food items.
- Acting as an antidote for cyanide poisoning.
| Related Links | |
| Aluminium chloride Formula | Aluminium fluoride Formula |
| Magnesium Iodide Formula | Inorganic Compound |
Sodium Nitride Formula FAQs
What is the chemical formula for Sodium Nitride?
How is Sodium Nitride structured at the atomic level?
What are the physical properties of Sodium Nitride?
Why is Sodium Nitride considered unstable?
What is the typical method for preparing Sodium Nitride?










