
Aluminium chloride formula, often spelled as aluminum chloride in the United States, is a chemical compound with the formula AlCl3. It is a significant and versatile inorganic compound known for its various industrial applications and importance in chemical processes. Aluminium chloride is a compound formed by the combination of aluminum (Al) and chlorine (Cl) atoms, and it exhibits unique properties and reactivity that make it a valuable component in various chemical reactions and industrial processes. Its chemical formula, AlCl3, reflects the composition of one aluminum atom bonded with three chlorine atoms, resulting in a highly reactive and useful compound.
Aluminium Chloride Formula
The chemical formula for aluminium chloride is AlCl3. This formula succinctly represents the composition of this inorganic compound. It consists of one aluminum (Al) atom and three chlorine (Cl) atoms bonded together.
Also Read: Hyponitrous Acid Formula
Aluminium Chloride Formula Name
The name "aluminium chloride" is derived from the elements it comprises, namely aluminum and chlorine. While the spelling "aluminium" is more commonly used in British English, the term "aluminum chloride" is prevalent in American English. It's important to note that the element aluminum (Al) is the most abundant metal in the Earth's crust, and chlorine (Cl) is a highly reactive non-metal, making their combination in aluminium chloride a noteworthy chemical compound.
Also Read: Nickel Chloride Formula
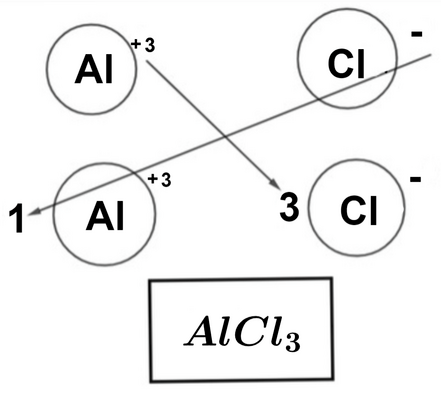
Aluminium Chloride Formula by Criss Cross Method
The criss-cross method, also known as the "swap and drop" method, is a simple technique used to determine the chemical formula of ionic compounds like aluminium chloride. Here's how it works:
1. Identify the valencies (charges) of the ions involved. Aluminum has a charge of +3 (Al³⁺), and chlorine has a charge of -1 (Cl⁻).
2. Swap the valencies, making the charge of one ion the subscript of the other, and vice versa. This results in Al³⁺ becoming the subscript for Cl, and Cl⁻ becoming the subscript for Al.
3. Write the chemical formula with the subscripts: AlCl₃.
So, by applying the criss-cross method, we determine that the formula of aluminium chloride is AlCl₃, indicating that there are three chlorine ions for every one aluminum ion in the compound.
Also Read: Lactic acid Formula
Aluminium Chloride Formula Equation
The formation of aluminium chloride can be represented by a balanced chemical equation. When aluminum reacts with chlorine, the following equation illustrates the process:
2Al + 3Cl₂ → 2AlCl₃
In this equation, two aluminum atoms react with three molecules of chlorine gas to produce two molecules of aluminium chloride. It is essential to balance the equation to ensure that the same number of each type of atom appears on both sides of the reaction.
Aluminium Chloride Formula Valency
Valency, also known as charge, is a measure of the ability of an element to combine with other elements to form compounds. In the case of aluminium chloride, aluminum (Al) has a valency of +3, meaning it can donate three electrons during chemical reactions. Chlorine (Cl) has a valency of -1 because it readily accepts one electron to achieve a stable electron configuration. The combination of Al³⁺ and Cl⁻ ions leads to the formation of the compound AlCl₃.
Aluminium Chloride Formula of Ionic Compound
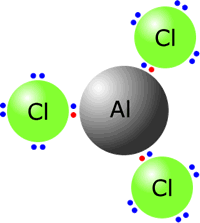
Aluminium chloride is classified as an ionic compound due to the strong electrostatic attraction between the positively charged aluminum ions (Al³⁺) and the negatively charged chlorine ions (Cl⁻). This ionic bonding results in forming a solid compound with distinct crystalline structures.
Aluminium Chloride Formula Compound
Aluminium chloride (AlCl3) is a compound with a white to pale yellow color and is highly hygroscopic, meaning it readily absorbs moisture from the air. It is commonly found as a hexahydrate (AlCl3·6H2O) in its hydrated form. The anhydrous form, AlCl3, is a powerful Lewis acid and finds application in various chemical processes, including the Friedel-Crafts reaction in organic synthesis, polymerization reactions, and as a catalyst in the production of various chemicals.
Aluminium chloride is a significant inorganic compound with the chemical formula AlCl3. Its name reflects its composition, consisting of aluminum and chlorine atoms. Understanding its formula, nomenclature, and the principles of its ionic bond formation is crucial for those engaged in chemistry, industrial processes, and scientific research. This versatile compound plays a crucial role in various applications, underscoring its importance in the field of chemistry.
| Related Links | |
| Pentane formula | Octane formula |
| Oxalic Acid formula | Nickel Chloride formula |
Aluminium Chloride Formula FAQs
Why is aluminum chloride formula AlCl3?
Is aluminium chloride Al2Cl3 or AlCl3?
What is the formula for aluminium chloride?
What is the valency of Al and Cl?










