Aluminium fluoride formula is a chemical compound of great significance in various industries and scientific applications, holds a unique place in the world of chemistry. In this article, we explore the fundamental aspects of aluminium fluoride, including its formula, nomenclature, chemical structure, and its critical role in many processes.
Aluminium Fluoride Formula
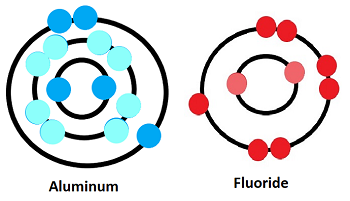
The chemical formula for aluminium fluoride is AlF3. This formula succinctly represents the composition of this inorganic compound. It consists of one aluminum (Al) atom bonded with three fluorine (F) atoms, forming a stable compound.
Aluminium Fluoride Formula Name
The name "aluminium fluoride" reflects its composition, comprising aluminium and fluorine elements. While it is often spelled as "aluminum fluoride" in the United States, "aluminium fluoride" is the more widely used term in British English.
Also Read: Lactic acid Formula
Aluminium Fluoride Formula Chemical Structure
Aluminium fluoride exhibits a simple chemical structure. It is a ionic compound composed of positively charged aluminum ions (Al³⁺) and negatively charged fluoride ions (F⁻). These ions are held together by strong electrostatic forces of attraction, forming a three-dimensional lattice structure.
Aluminium Fluoride Formula Unit
The aluminium fluoride formula is AlF3. This indicates that in the crystal lattice of the compound, there is one aluminum ion (Al³⁺) surrounded by three fluoride ions (F⁻), ensuring electrical neutrality.
In aluminium fluoride formula, the aluminum ion (Al³⁺) has a charge of +3, signifying the loss of three electrons to achieve a stable electron configuration. On the other hand, the fluoride ion (F⁻) carries a charge of -1, indicating its acceptance of an additional electron to attain stability. The combination of these oppositely charged ions ensures the compound's electrical neutrality.
Aluminum Fluoride Formula Compound
Aluminium fluoride (AlF3) is an inorganic compound. It is a white, crystalline solid that is highly refractory and exhibits excellent chemical stability. This compound finds extensive use in various industrial processes, such as aluminum smelting, the production of synthetic cryolite (Na3AlF6), and as a flux in the manufacture of ceramics and glass.
Also Read: Formic Acid Formula
Aluminum Fluoride Formula Weight
The molar mass of aluminium fluoride formula (AlF3) can be calculated by summing the atomic masses of its constituent elements: one aluminum atom (Al) and three fluorine atoms (F). The molar mass of AlF3 is approximately 83 grams per mole (g/mol).

Aluminum Fluoride Formula Electrically Neutral
The ionic compounds, such as aluminium fluoride formula, are electrically neutral overall. This means that the negative charges of the fluoride ions balance the positive charges of the aluminum ions. In AlF3, one Al³⁺ ion and three F⁻ ions come together so that their charges cancel out, resulting in a neutral compound.
Aluminium Fluoride Equation
The formation of aluminium fluoride is a result of a chemical reaction between aluminum and fluorine:
2Al + 3F2 → 2AlF3
In this balanced equation, two aluminum atoms react with three molecules of fluorine gas to produce two molecules of aluminium fluoride. This reaction is exothermic, releasing a significant amount of energy, and it plays a pivotal role in the production of aluminium and other industrial applications.Aluminum fluoride (AlF3) is an inorganic compound with a chemical formula that reflects its composition of one aluminum atom bonded with three fluorine atoms. Its chemical structure, electrical neutrality, and chemical properties make it indispensable in various industrial processes, particularly in aluminum production. Understanding the formula and significance of aluminium fluoride is key to appreciating its essential role in chemistry and industry.
Also Read: Octane Formula
Physical Properties of Aluminium Fluoride
- In addition to being white powder or granules, aluminum fluoride is odorless. It is also denser than water.
- The solubility of this compound in water is approximately 559g/100mL at 25°C.
- In addition, this molecule is very marginally soluble in alkalis and acids.
- Aluminium fluoride is less affected by even highly concentrated acids
- The melting point of aluminum fluoride formula is 1291 degrees Celsius.
- The vapor pressure of this substance is 1 mm at 1238 °C.
- The chemical is less prone to oxidation than trialkyl aluminium.
- Aluminum fluoride is non-flammable in nature.
- Hydrogen fluoride vapors are generated when this chemical breaks down.
- Sublimation of aluminum fluoride takes place at 1272 °C and 760 mmHg pressure.
Chemical Properties of Aluminium Fluoride
In addition to AlF3, experts are familiar with various other hydrates of aluminum fluoride. These compounds, which have the formula AlF3.xH2O, include a monohydrate (x = 1), two polymorphs of a trihydrate (x = 3), a hexahydrate (x = 6), and a nonahydrate (x = 9). Most aluminum fluoride is produced by reacting alumina with hydrogen fluoride at a temperature of 700 °C. Alternatively, fluorosilicic acid can also be utilized to produce aluminum fluoride. Another option is to thermally decompose ammonium hexafluoroaluminate to obtain aluminum fluoride.
| Related Links | |
| Glycerol Formula | Hypochlorous Acid Formula |
| Azelaic Acid Formula | Butan 1 ol Formula |
Aluminium Fluoride Formula FAQs
What is the name of AlF3?
What is aluminium fluoride used for?
What is AlF3 chemistry?
What type of bond is AlF3?