
The magnesium iodide formula is MgI2. Magnesium iodide is an ionic compound formed by the combination of magnesium (Mg) and iodine (I) atoms. In the Magnesium Iodide compound, magnesium loses two electrons to become a Mg²⁺ ion, while iodine gains one electron to form I⁻ ions. The ionic bonding between these positively charged magnesium ions and negatively charged iodine ions leads to the formation of solid magnesium iodide, which is often used in various chemical and industrial applications.
Magnesium Iodide Formula
Magnesium iodide formula is a chemical compound formed by the combination of magnesium (Mg) and iodine (I). Its chemical formula is represented as MgI2. This formula is essential in defining the composition and stoichiometry of magnesium iodide, a compound with various applications in chemistry and industry.
Also Read: Ionic Compound Formula
Magnesium Iodide Formula by Criss Cross Method
The criss-cross is a simple technique for determining the chemical formula of ionic compounds like Magnesium Iodide Formula. To find the formula for magnesium iodide using this method, you cross the numerical charges of the ions. Magnesium ions carry a +2 charge (Mg²⁺), and iodine ions have a -1 charge (I⁻). By crossing these charges, you get the formula MgI2. This formula showcases the balanced ratio of ions required for the compound's formation.Also Read: Lead (II) Chloride Formula
Magnesium Iodide Formula and Charge
In magnesium iodide, the magnesium ion (Mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron configuration. On the other hand, the iodine ion (I⁻) carries a negative charge of -1, signifying the gain of an electron. This difference in charge is the driving force for the ionic bonding between the two elements, leading to the formation of magnesium iodide.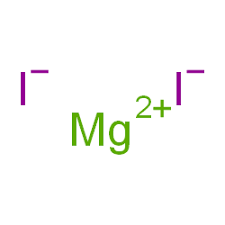
Also Read: Butane Formula
Magnesium Iodide Formula Ionic
The formula MgI2 highlights the ionic nature of magnesium iodide. Ionic compounds are formed through the transfer of electrons from one element to another. In this case, magnesium, a metal, transfers two electrons to iodine, a non-metal, to create Mg²⁺ and I⁻ ions. The strong electrostatic attraction between these oppositely charged ions results in the formation of an ionic lattice, where electrostatic forces hold together the positive and negative ions.Magnesium Iodide Formula Molecular
While the chemical formula of magnesium iodide (MgI2) indicates its ionic composition, it's essential to recognize that this compound is typically found as an ionic solid in its crystalline form. In the solid state, it doesn't exist as individual molecules but as a three-dimensional array of Mg²⁺ and I⁻ ions. However, if magnesium iodide were to be melted or vaporized, it would exist as discrete MgI2 molecules.Magnesium Iodide Formula Structure
Its ionic nature primarily determines the structure of magnesium iodide (MgI2). Here is an overview of its structure: Magnesium iodide is an ionic compound that consists of negatively charged ions (anions) and positively charged ions (cations). During the formation of MgI2, magnesium (Mg) atoms lose two electrons each to form Mg2+ cations, while iodine (I) atoms gain one electron each to form I anions. A three-dimensional crystal lattice structure is formed in magnesium iodide when it is solid. In the solid, the Mg2+ cations and I anions alternate, creating a repeating pattern. Within the crystal lattice, each magnesium ion (Mg2+) is surrounded by six iodine ions (I) in an octahedral coordination geometry. This means that every magnesium ion is bonded to six iodine ions. The ionic lattice structure of magnesium iodide is densely packed due to the strong electrostatic attraction between positively charged magnesium ions and negatively charged iodine ions. There are no discrete molecules of magnesium iodide in its solid state. While its chemical formula is MgI2, it is found in the form of a solid crystal as a continuous ionic lattice. The discrete MgI2 molecules can only be found when magnesium iodide is heated or vaporized. A classic example of an ionic compound is magnesium iodide, in which the attractive forces between them determine the arrangement of positively and negatively charged ions in a crystal lattice. Its unique physical and chemical properties are derived from this structure.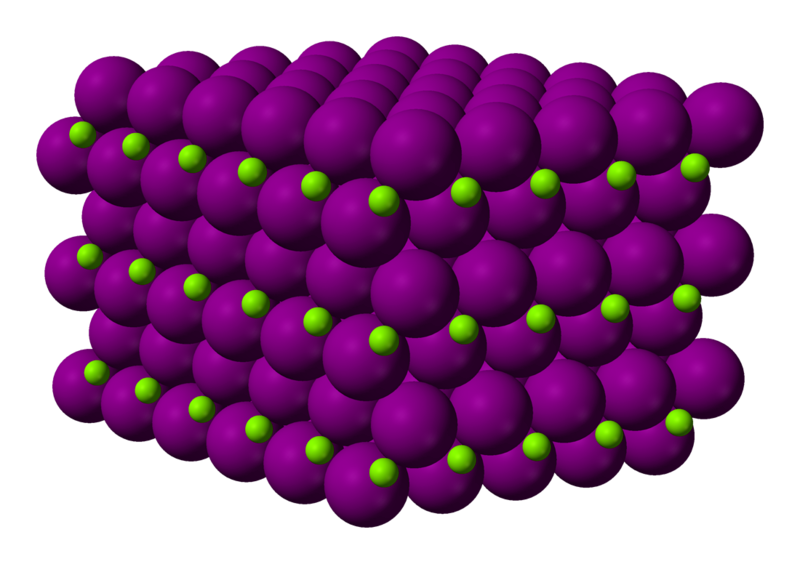
Magnesium Iodide Formula and Names
Magnesium iodide, with its chemical formula MgI2, is sometimes referred to by its systematic IUPAC name, which is "Magnesium Diiodide." This name conveys its composition clearly, indicating the presence of two iodine atoms for every magnesium atom. Additionally, magnesium iodide may be known by its common name, which is widely used in chemical literature and practical applications. Understanding the formula and names of magnesium iodide is essential for accurate communication in the field of chemistry and its various applications.Properties of Magnesium Iodide
Magnesium iodide formula (MgI2) is an ionic compound with distinct properties that make it useful in various applications. Here are some of its key properties:Physical State: Magnesium iodide is a white crystalline solid at room temperature. It is hygroscopic, meaning it readily absorbs moisture from the air, which can affect its physical appearance and properties.
Solubility: Magnesium iodide is soluble in water and other polar solvents. When dissolved in water, it forms a clear solution. The solubility of magnesium iodide in water increases at higher temperatures.
Melting and Boiling Points: Magnesium iodide has a high melting point, indicating its stability at high temperatures. It decomposes before reaching its boiling point due to the high reactivity of iodine.
Electrical Conductivity: In its molten state or when dissolved in water, magnesium iodide can conduct electricity because it dissociates into ions. It does not conduct electricity in its solid state as the ions are held in a fixed lattice structure.
Hygroscopic Nature: Magnesium iodide has a strong affinity for water and readily absorbs air moisture. This hygroscopic nature can affect its stability and make it challenging to handle in humid environments.
Chemical Reactivity: Magnesium iodide is reactive, especially with oxygen and moisture. It reacts with oxygen in the air to form magnesium oxide and iodine. Therefore, it is usually stored in airtight containers to prevent exposure to moisture and air.
Uses: Magnesium iodide finds applications in various fields. It is used in the preparation of magnesium metal, in manufacturing some types of fluorescent lamps, and as a reagent in organic synthesis. It is also used in certain medical imaging procedures and as a source of iodine in animal feeds and nutritional supplements.
Understanding these properties is essential for handling magnesium iodide sReafely and for its effective use in specific industrial and chemical processes.| Related Links | |
| Acetone formula | Amino Acid Formula |
| Actetamide formula | Ammonium Acetate Formula |
Magnesium Iodide Formula FAQs
How do you write magnesium iodide
What state is magnesium iodide?
What is the product of MgI2?
What color is magnesium iodide?










