
science class 10th chapter-Chemical Reaction and equation Formula & Important points
Jul 27, 2022, 16:45 IST
Difference between Physical change and Chemical change
| S.NO. | PHYSICAL CHANGE | S.NO. | CHEMICAL CHANGE |
| (i) |
Those changes in which no new substances
are formed are called physical changes |
(i) |
Those changes in which the original substances
lose their chemical nature and identity and form new chemical substances with different properties are called chemical changes |
| (ii) | It is a temporary change | (ii) | It is a permanent change |
| (iii) | It is easily reversible | (iii) | It is usually irreversible |
| (iv) |
In a physical change the mass of
substance does not alter |
(iv) |
In a chemical change the mass of
the substance does alter |
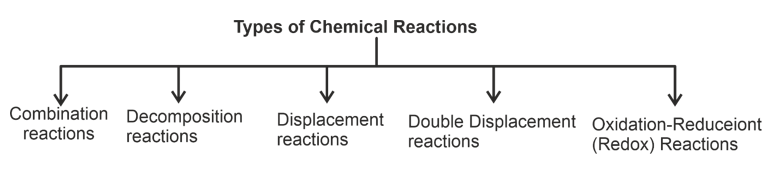
Types of Chemical Reactions:
- Addition or combination reactions: Two or more substances combine to form a single substance.
CaO+Co 2 →CaCO 3
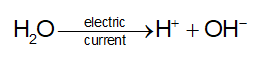
- Decomposition reaction: One chemical substance splits to give two or more substances either by heat energy (Thermolysis) or light (Photolysis) or by electricity (Electrolysis).
Thermolysis
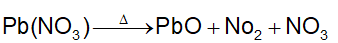
Electrolysis
Photolysis
2AgCl→2Ag+Cl 2
- Displacement Reaction: More reactive element displaces less reactive element from its compound or salt.
Fe+CuSO 4 →FeSO 4 +Cu
- Double Displacement Reaction: Two elements interchange their respective salts or ions to form new compounds.
FeCl 3 +CuSO 4 →FeSO 4 +CuCl 2
Academic team of Physics Wallah prepared NCERT Solutions do solve all questions given in Exercise with the help of NCERT solutions for class 10 Science . If any students need to take the online test to check their concepts or understanding then they can visit Quiz for Chemical Reaction and Equation .
Download the Free Pdf sheet of Chemical Reaction and equation it consists of Short notes and key points of Chemistry for class 10 from the link given below.









