
Modern Periodic Table : The Modern Periodic Table is a remarkable scientific tool that has revolutionized our understanding of the elements and their properties. Modern Periodic Table was proposed by Henry Moseley. The Modern Periodic Table organises the elements based on their atomic number, paving the way for a deeper comprehension of the fundamental building blocks of matter.
The key feature of the Modern Periodic Table is its systematic arrangement of elements based on their atomic number, which is the number of protons in an atom's nucleus. This organization reflects the periodicity in the properties of elements, revealing trends and patterns that assist scientists in predicting the behaviour of different elements.
The synthesis of synthetic elements in laboratories has expanded the Periodic Table, pushing the boundaries of our knowledge of the elements. Despite these changes, the fundamental principles of the Modern Periodic Table have remained intact, providing a stable foundation for scientific inquiry.
-
It was proposed by Henry Moseley.
-
The modern periodic table is based on atomic number.
-
Moseley did an experiment in which he bombarded high speed electron on different metal surfaces and obtained X-rays. He found out that
where v = frequency of X-rays From this experiment, Moseley concluded that the physical and chemical properties of the elements are periodic function of their atomic number . It means that when the elements are arranged in the increasing order of their atomic number, elements have similar properties after a regular interval. This is also known as ‘Modern periodic Law’ .
Modern Periodic Law: The physical and chemical properties of elements are a periodic function of the atomic number.
Long form/Present form of modern Periodic Table
(It is also called as ‘Bohr, Bury, Rang & Werner Periodic Table)(i) It is based on the Bohr-Bury electronic configuration concept and atomic number.
(ii) This model is proposed by Rang & Werner
(iii) In long form of periodic table, elements are classified into 7 periods and 18 groups
(iv) According to I. U. P. A. C. 18 vertical columns are named as 1 st to 18 th group.
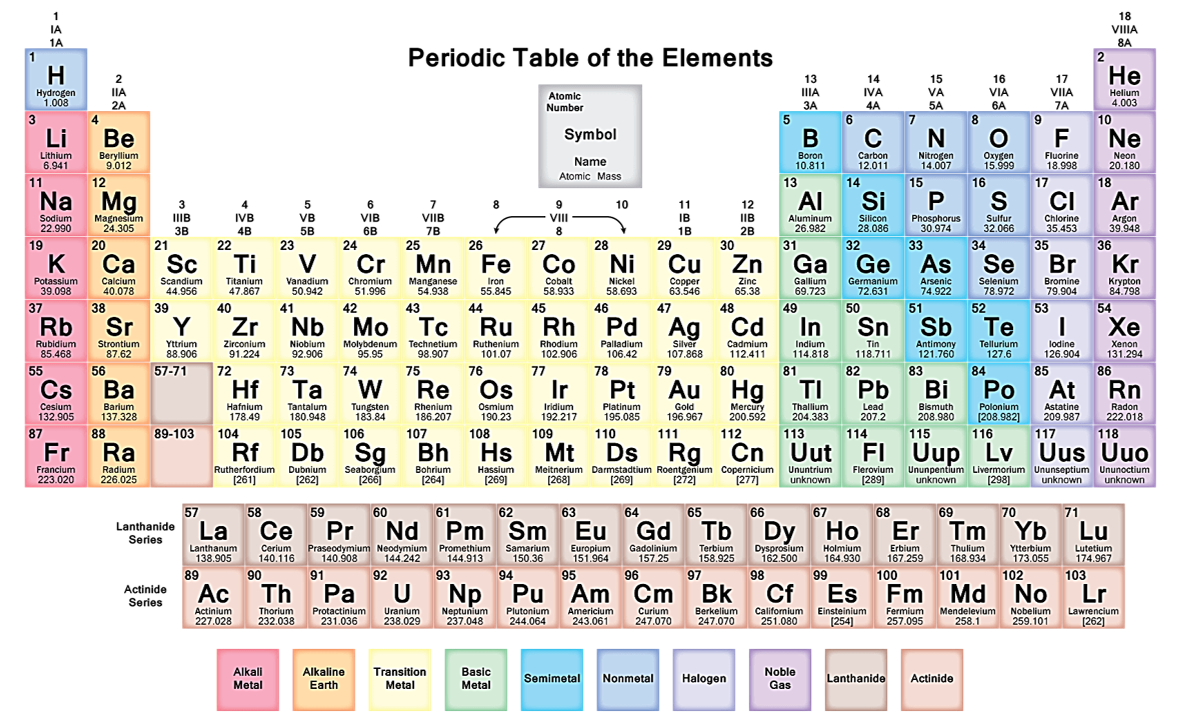
(v) The co-relation between the groups in long from of periodic table and in modern form of periodic table are given below:
|
IA |
IIA |
IIIB |
IVB |
VB |
VIB |
VIIB |
VIII |
IB |
IIB |
IIIA |
IVA |
VA |
VIA |
VIIA |
0 |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 9 10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
(vi) Elements belonging to same group have same number of electrons in the outermost shell, so their properties are similar.
|
Period |
n |
Sub shell |
No. of elements (Magic Number) |
Element |
Name of Period |
|
1. |
1 |
1s |
2 |
1 H – 2 He |
Shortest |
|
2. |
2 |
2s, 2p |
8 |
3 Li – 10 Ne |
Short |
|
3. |
3 |
3s, 2p |
8 |
11 Na – 18 Ar |
Short |
|
4. |
4 |
4s, 3d, 4p |
18 |
19 K – 36 Kr |
Long |
|
5. |
5 |
5s, 4d, 5p |
18 |
37 Rb – 54 Xe |
Long |
|
6. |
6 |
6s, 4f, 5d, 6p |
32 |
55 Cs – 86 Rn |
Longest |
|
7. |
7 |
7s, 5f, 6d, 7p |
32 |
87 Fr – 118 Uuo |
Complete |
Modern Periodic Table FAQs
Q.1 : What is the long form of the modern periodic table?
Q.2 : Who contributed to the development of the long form of the periodic table?
Q.3 : Why is the present form of the periodic table considered more accurate and reliable?
Q.4 : How are elements arranged in the Modern Periodic Table?
Q.5 : What are periods and groups in the Modern Periodic Table?
Q.6 : What are the main features of the Modern Periodic Table?










