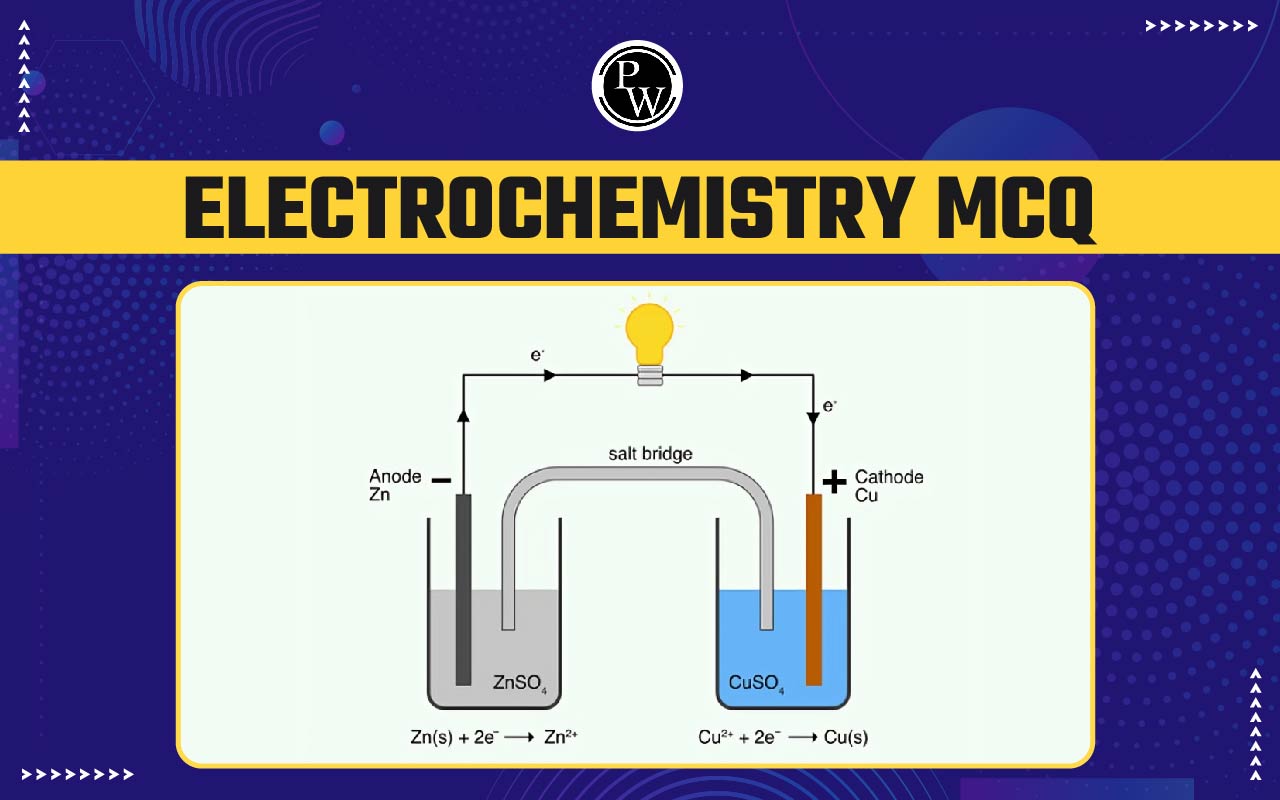
Electrochemistry MCQ: Electrochemistry is an essential chapter in class 12 chemistry that studies how chemical reactions produce and use electrical energy. For NEET preparation, the Electrochemistry NEET MCQ consists of multiple-choice questions designed to test your knowledge of these reactions, electrochemical cells, and standard electrode potentials.
The Electrochemistry Class 12 MCQ set includes questions specifically designed for the Class 12 Chemistry curriculum. These questions help students revise and understand important electrochemistry concepts. Electrochemistry MCQ NCERT questions are aligned with the NCERT curriculum, focusing on key topics from the textbook. This resource supports classroom learning and exam preparation.Electrochemistry MCQ for NEET
Electrochemistry MCQ with Answers offers a structured format for accessing and reviewing multiple-choice questions and their solutions. Electrochemistry MCQ PDF is a useful study tool for practicing and preparing effectively for exams. The Electrochemistry Class 12 MCQ set includes questions specifically designed for the Class 12 Chemistry curriculum. These questions help students revise and understand important electrochemistry concepts.| NEET Chemistry Important Links | ||
|---|---|---|
| NEET Chemistry Syllabus | NEET Chemistry Notes | NEET Chemistry Chapter wise Weightage |
Electrochemistry MCQ Class 12 Chemistry
Electrochemistry MCQ Class 12 Chemistry covers important concepts from the Class 12 Chemistry syllabus. Topics include electrochemical cells, Faraday’s laws of electrolysis, and the electrochemical series. These questions help students review and prepare for their Class 12 exams and NEET. Electrochemistry MCQ Class 12 provides practice questions to help students prepare for their Class 12 exams. These questions focus on crucial electrochemistry concepts to aid in the review and consolidation of knowledge.Also Check:
Electrochemistry NEET Questions With Solutions
Electrochemistry NEET Questions With Solutions resource provides multiple-choice questions along with detailed answers and explanations. This helps students understand the principles of electrochemistry better and improves their problem-solving skills for the NEET exam. The Electrochemistry MCQ NEET set is designed for students preparing for the NEET exam. It covers essential electrochemistry topics that are often tested, helping students improve their exam readiness and confidence.1. Which of the following statements is correct about the galvanic cell?
- It converts chemical energy into electrical energy.
- It converts electrical energy into chemical energy.
- It converts metal from its free state to its combined state.
- It converts electrolytes into individual ions.
2. A galvanic cell consists of:
- a cadmium cell
- two half cells
- three half cells
- Lead accumulation.
3. A device used to convert chemical energy into electrical energy is called________.
- An electrolytic cell
- Voltaic cell
- A coulometer.
- A Voltagemeter
4. In a galvanic cell, the salt bridge is used to:
- To produce current at a constant strength.
- Facilitate the continuity of the cell reaction.
- Prevent the accumulation of charges around the electrodes.
- All of these
5. In a galvanic cell, the reactions taking place in the anodic half-cell and the cathodic half-cell will be:
- Reduction
- Oxidation
- Oxidation and reduction
- Reduction and oxidation
6. In a galvanic cell, electron flow will be from:
- Negative electrode to positive electrode
- Positive electrode to negative electrode
- There will be no flow of electrons
- Cathode to anode in the external circuit
7. In voltaic cells, the electrons are lost during the oxidation flow from:
- Cathode to anode
- Anode to cathode
- Remains as it is in the electrolytic solution
- Electrons flow from the electrolytic solution to the electrode
8. In galvanic cell:
- Oxidation occurs at the anode
- Reduction occurs at the cathode
- Chemical energy is converted into electrical energy
- All of these
9. Which of the following statements is correct for galvanic cells written in an abbreviated form?
- The left electrode is a cathode
- The right electrode is an anode
- The left electrode is the positive terminal
- The right electrode is the positive terminal
10. Which of the following is not true of a galvanic cell represented in the IUPAC system?
- The right-hand electrode is a +ve terminal.
- The right-hand electrode acts as a cathode.
- Electrons are given out in the external circuit from the anode.
- Electrons are given out in the external circuit from the cathode
11. In a galvanic cell, by which the negative ions are displaced?
- Electrode [2013]
- Salt bridge
- External path of copper wire
- Electron
12. Statement-I: The salt bridge is used generally in electrochemical cells.
Statement II: The ions of the electrolyte used in the salt bridge should have nearly the same transport numbers.
- Both Statement I and Statement II are correct.
- Both Statement I and Statement II are incorrect.
- Statement I is correct and Statement II is incorrect.
- Statement I is incorrect and Statement II is correct.
13. A salt bridge is used because it:
- maintains the electrical neutrality of the two half-cells.
- enhances the flow of electrons by overcoming the liquid junction potential.
- provides the lost ions.
- forms insoluble precipitates with the electrolytes in the two half-cells.
14. If a salt bridge is removed between the two half cells, the voltage:
- drops to zero
- does not change
- increases rapidly
- increases gradually
15. The incorrect statement is:
- The salt bridge connects two half-cells.
- The salt bridge maintains electrical neutrality.
- Salt bridge minimizes liquid junction potential.
- Salt bridge increases the EMF of the cell.
16. Daniell's cell is:
- an irreversible voltaic cell
- reversible voltaic cell
- a reversible secondary cell
- irreversible secondary cell
17. In a Daniell cell,
- The chemical energy liberated during the redox reaction is converted to electrical energy.
- The electrical energy of the cell is converted to chemical energy.
- The energy of the cell is utilized in the conduction of the redox reaction.
- The potential energy of the cell is converted into electrical energy.
18. Which of the following is incorrect?
- Zinc acts as an anode in the Daniell cell.
- In a Li-Zn couple, zinc acts as a cathode.
- Iron will displace copper in the solution.
- Tin displaces zinc in solution.
19. The electrode with the highest reduction potential is written at:
- The extreme left
- The extreme right
- In the middle
- Anywhere
20. Which of the following statements is correct?
- The cathode is -ve terminal in both galvanic and electrolytic cells
- The anode is +ve terminal in both galvanic and electrolytic cells
- The cathode and anode are -ve terminals in an electrolytic and galvanic cell.
- The cathode and anode are +ve terminals in electrolytic and galvanic cells.
21. In a galvanic cell external potential is applied alternately as 1.0 V and 1.2 V then it behaves as a .......... and ........... cell.
- Electrochemical cells and electrolytic
- Electrolytic cell and electrochemical cell
- Both potential electrochemical cell
- Both potential electrolytic cell
22. Which of the following is correct?
- The cathode is a positive terminal in an electrolytic cell
- The cathode is a negative terminal in a galvanic cell
- Reduction occurs at the cathode in each of the cells
- Oxidation occurs at the cathode in each of the cells
23. The following statements are correct with respect to both electrolytic cells and galvanic cells;
- In both cells, the anode is shown by the +ve sign.
- In both cells, the cathode is shown by the -ve sign.
- In both cells, a reduction reaction takes place at the cathode.
- In both cells, an oxidation reaction takes place at the cathode
24. Assertion (A): The Anode is the electrode at which oxidation occurs and the cathode is the electrode at which reduction occurs.
Reason (R): Anode and cathode in electrochemical cells and electrolytic cells have opposite polarities.
- Both Assertion (A) and Reason (R) are correct and Reason (R) is the correct explanation for Assertion (A).
- Both Assertion (A) and Reason (R) are correct but Reason (R) is not the correct explanation for Assertion (A).
- Assertion (A) is correct but Reason (R) is incorrect.
- Both Assertion (A) and Reason (R) are incorrect.
25. Which of the following statements about galvanic and electrolytic cells is correct?
- The electrons in the external wire flow from the cathode to the anode in an electrolytic cell.
- Oxidation occurs at the cathode of both cells.
- Both (1) and (2)
- None of them.
26. The electrode potential measures:
- Tendency of the electrode to gain or lose electrons.
- Electron affinity for elements.
- Difference in the ionization potential of electrode and metal ion.
- Heat from combustion.
27. The cathodic standard reduction potential minus anodic standard reduction potential is equal to:
- Faraday
- Coulomb
- Cell potential
- Ampere
28. A standard hydrogen electrode has a zero potential because:
- Hydrogen can be most easily oxidized.
- The electrode potential is assumed to be zero.
- Hydrogen has only one electron.
- Hydrogen is the lightest element.
29. The standard hydrogen electrode potential is 0.0 V. How can it be formed?
- Experimentally
- According to the definition,
- Cu is taken as a cathode and calculation is done
- Zn is taken as an anode and calculation is done
Electrochemistry MCQ FAQs
What is covered in the Electrochemistry NEET MCQ?
How can the Electrochemistry MCQ Class 12 Chemistry help me?
Where can I find detailed solutions for Electrochemistry NEET Questions?
What is included in the Electrochemistry MCQ with Answers PDF Download?
How do Electrochemistry MCQ NCERT questions help with exam preparation?