
Acetaldehyde , also known as MeCHO or ethanal, is an organic chemical compound with a molecular formula of C2H4O (CH3CHO). It is a colorless liquid that has a pungent odor and is soluble in water. It can cause mucous irritation due to its narcotic action and damage the liver when alcohol is consumed. Acetaldehyde can be found in perfumes, drugs, flavoring agents, dyes and other products; however, it is toxic when applied externally for extended periods. This compound is mainly used as an intermediate in acetic acid production.
Acetaldehyde is an organic compound with the chemical name C2H4O. Acetaldehyde is also known as MeCHO. It has no color and is flammable. It has a suffocating odor. It is non-corrosive. Acetaldehyde is miscible with naphthalene, gasoline, xylene, ether, turpentine, alcohol, and benzene.
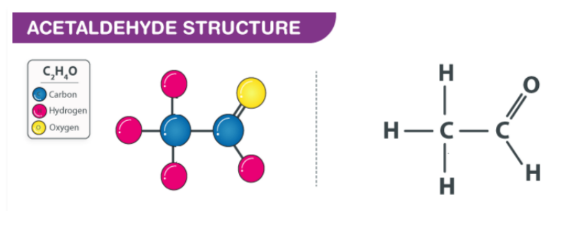
Acetaldehyde Structure (C2H4O Structure)
The molecular formula for acetaldehyde is CH3CHO, or C2H40, with a molar mass of 44.053 g mL -1 . It has the characteristic functional group of an aldehyde (H-C=O) linked to a methyl group (-CH3), which makes acetaldehyde the second least complex aldehyde after formaldehyde. The C atom from the aldehyde has hybridization sp2, while the methyl group has sp3, creating a planar-trigonal and tetrahedral geometry in the molecule. A visual representation is displayed below.
Acetaldehyde Occurrence
As an intermediate in the ethanol metabolism, acetaldehyde is produced by the enzyme alcohol dehydrogenase, which transforms ethanol into this compound.
Preparation of Acetaldehyde
The Wacker process, which uses copper or palladium as the catalyst, is another method for the hydration of acetylene or ethylene today:
2CH2=CH2 + O2 → 2CH3CHO
Acetaldehyde was previously produced by the hydration of acetylene using mercury (II) salts as a catalyst:
C2H2 + Hg2+ + H2O → CH3CHO + Hg
When the reaction is conducted at 90-95oC, the acetaldehyde is separated from the mercury and water and cooled to 25-30oC. In the presence of copper as a catalyst, partial dehydrogenation of ethanol produces acetaldehyde as another method:
CH3CH2OH → CH3CHO + H2
The ethanol vapor is passed at 260-290°C during this process.
Also Check – Aluminium Nitrate Formula
Acetaldehyde Physical Properties
In addition to being colorless and having a strong odor, acetaldehyde boils at 20.20C and melts at -123C. It has a density of 0.784 g mL-1. Acetaldehyde is soluble in miscible water, ethanol, benzene, acetone, and toluene and is slightly soluble in chloroform
| C2H4O | Acetaldehyde |
| Molecular Weight/ Molar Mass | 44.05 g/mol |
| Density | 0.784 g/cm3 |
| Boiling Point | 20.2 °C |
| Melting Point | -123.5 °C |
Chemical Properties of Acetaldehyde
This organic compound has properties similar to formaldehyde, making it an essential precursor in many organic syntheses. Through a condensation reaction, we can gain pentaerythritol, an intermediate utilized in explosive production, and ++ other hydroxyethyl derivatives when a Grignard reagent is used. Additionally, acetaldehyde is a critical component in creating heterocycles like imines and pyridines.
Also Check – Elevation of Boiling Point Formula
Uses of Acetaldehyde
The chemical industry has a broad range of usages for acetaldehyde. It is often used to manufacture dyes, fragrances, acetic acid, and flavoring agents. Additionally, it can feature in fuel composition and as a solvent in industrial processes such as papermaking, leather tanning and rubber production. By combining with urea, the resin ethylidene diacetate is produced - a precursor to vinyl acetate, which is itself used to produce polyvinyl acetate. Previously, it was also employed in the production of n-butyraldehyde, though today, propylene has largely taken its place.
Also Check – Ammonium Nitrate Formula
Safety Hazards and Effects on Health
In addition to being toxic, acetaldehyde also irritates the mucous membranes, and large doses can be fatal, causing respiratory paralysis. Lastly, it is highly flammable and can form peroxides.
Acetaldehyde Toxicity
Its first metabolite, acetaldehyde, is responsible for at least part of ethanol toxicity, and it is significantly more potent than ethanol. Acetaldehyde gets into your bloodstream and damages your membranes and possibly scars. Acetaldehyde intoxication has the greatest impact on the brain. It impairs memory and causes problems with brain activity. It also causes a hangover, a racing heart, and a headache.
Based on inadequate human cancer research and animal studies that have uncovered nasal cancers in rats and laryngeal tumours in hamsters, acetaldehyde is considered to be a possible human carcinogen. Acetaldehyde plays a major role in allowing alcohol to cause fibrogenesis and mutation in the liver. It causes adducts which deteriorate the functionality of proteins such as enzymes and damages DNA, which may lead to mutagenesis.
Acetaldehyde Formula FAQs
Q1. What is acetaldehyde and where is it found?
Q2. Is acetaldehyde harmful to human health?
Q3. How is acetaldehyde used in industry?
Q4. What are the sources of acetaldehyde in indoor air pollution?
Q5. How is acetaldehyde metabolized in the body?










