
In organic chemistry, acetonitrile is the simplest nitrile. The manufacture of acrylonitrile mainly produces it. It is also used for organic synthesis and butadiene purification. In 1847, the French chemist Jean-Baptiste Dumas made the first preparation of Acetonitrile.
Acetonitrile Formula
The chemical formula of acetonitrile is CH3CN, and its molar mass is 41.05 g/mol. Scientists categorize it as a nitrile, CN, composed of one carbon atom triple bonded to a nitrogen atom and with a methyl group (-CH3) of three hydrogen atoms attached. The bond angle between the methyl group carbon, the central atom, and the nitrogen atom is 180°. Thus, its condensed formula is C2H3N, with its corresponding chemical structure being:
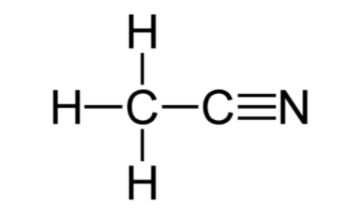
Structure Of Acetonitrile - C2H3N or CH3CN
According to organic chemistry, a nitrile is a carbon atom with a triple bond to a nitrogen atom. Acetonitrile is the simplest organic nitrile because it contains a carbon-nitrogen triple bond.
Acetonitrile Occurrence
During the process of manufacturing acrylonitrile, it forms a byproduct. It typically exists in aqueous solution since it is an acetic acid derivative. Also, it is a secondary product used to find acrylonitrile from propylene ammoxidation or waste streams used in extraction and chromatography. In addition to benzene, alcohol, and methanol, we can also find other compounds.
Acetonitrile Preparation
Acrylonitrile can be obtained as a byproduct through manufacturing or synthesized via hydrogenation of carbon monoxide mixtures or dehydration of acetamide and ammonia. An alternate method for preparing pure Acetonitrile consists of two steps: neutralizing acetic acid and ammonia to create ammonium acetate, then mixing an aqueous solution of this compound with gaseous ammonia, preheating the mixture, and passing it through a fixed bed reactor filled with a sizeable aluminum oxide catalyst for the reaction. Once the Acetonitrile is created in gas form, it must be refined continuously to render it in its purest form.
Also Check – Ammonium Nitrate Formula
Acetonitrile Physical Properties
Physically, it appears as a colorless clear liquid with an aromatic odor. It is a weak base with a density of 0.783 g/cm3. Its melting point is between -46 and -44 oC, while its boiling point is between 81.3 and 82.1 oC. It is miscible with water.
Acetonitrile Chemical Properties
It is a nitrile (hydrogen cyanide) with a methyl group instead of hydrogen. Additionally, it is a polar compound, meaning its atoms can attract electrons to themselves. Furthermore, the nitrogen atom has a greater electronegative charge than the carbon atom.
Also Check – Elevation of Boiling Point Formula
Acetonitrile Uses
A primary purpose of it is to serve as a two-carbon building block in organic synthesis. Chemists use it when constructing large and complex molecules as a starting material. Additionally, it allows two carbon atoms and nitrogen atoms to be added to the molecule. Acetonitrile is primarily used as a solvent for reactions and compound purification.
Due to its polar nature, the compound can successfully solvate a wide range of organic compounds. Furthermore, the high boiling point makes it ideal for conducting reactions at high temperatures. In addition, it is highly flammable, liquid, vapor, and has acute toxicity when it comes in contact with skin. In addition, it can cause serious eye damage and irritation.
Among its most common applications is compound purification using high-performance liquid chromatography (HPLC), an analytical chemistry application. Additionally, it has been used in nail polish remover formulations. Pharmaceuticals, rubber products, perfumes, pesticides, and batteries are also manufactured using them.
Also Check – Chemistry Formula For Solubility Product
Safety and Health Hazards
Small amounts of acetonitrile are relatively toxic, but in large doses, it can be converted into hydrogen cyanide, which is toxic. Since it takes time for the body to metabolize acetonitrile to cyanide, its toxic effects are delayed. The eyes, throat, lungs, and nose can become irritated.
Its long-term effects are thyroid enlargement, headaches, numbness, lack of appetite, dizziness, weakness, and tremors. Scientists observe animal reproductive problems, including birth defects and low birth weights. Acetonitrile can affect the liver, lungs, central nervous system, and kidneys for a long time.
Acetonitrile Formula FAQs
Q1. What is the chemical formula for acetonitrile?
Q2. Is acetonitrile toxic to humans?
Q3. What is the role of acetonitrile in chromatography?
Q4. Can acetonitrile be used as a solvent in the pharmaceutical industry?
Q5. Are there any safety precautions when working with acetonitrile?










