
The Solubility Product formula Constant Ksp is the equilibrium constant for dissolving solids into aqueous solutions. Ksp is an equilibrium constant whose value varies with temperature. Ksp increases as temperature increases since solubility increases.
Generally, solubility is the ability of a substance to dissolve in a solvent to form a solution. Ionic compounds (which dissociate into cations and anions) vary significantly in their solubility in water. It is possible for some compounds to absorb moisture from the atmosphere and become highly soluble, whereas others are highly insoluble.Also read : Surface Chemistry Formula
Solubility Product Definition
The definition of the solubility product is as follows: Solubility is the property of a substance known as a solute to get dissolved in a solvent to form a solution. Ionic compounds dissociating in water and forming anions and cations vary widely in their solubility. Some compounds are highly soluble and absorb atmospheric moisture, but others are insoluble.Solubility Product Formula
Saturated solutions of ionic compounds with relatively low solubility are said to be in a state of dynamic equilibrium between the ionic compound and the undissolved solid. The Ksp formula is given in the form of the following equation: [caption id="attachment_16300" align="alignnone" width="300"]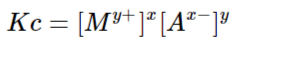
Significance of Solubility Product
Many parameters affect solubility, including the lattice enthalpy of salts and the solvation enthalpy of ions in solutions.- As salts dissolve in a solvent, the interactions between ions and the solvent have to overcome the strong forces of attraction of the solute (lattice enthalpy of its ions).
- As ions solvate, their enthalpy is always negative, which means that energy is released.
- Solvation enthalpy is determined by the nature of the solvent used during solvation.
- Solvents without polar groups have a small solvation enthalpy, which is insufficient to overcome the lattice enthalpy.
- Thus, salts cannot be dissolved in non-polar solvents. Therefore, for salt to be dissolved in a solvent, its solvation enthalpy should be greater than its lattice enthalpy.
- Each salt has a different solubility depending on its temperature.
Also Check - Ammonium Nitrate Formula
Salts are Classified According to their Solubility, which is Given in the Table :| Category I | Soluble | Solubility is > 0.1 |
| Category II | Moderately Soluble | Solubility <0.1M |
| Category III | Highly Soluble | Solubility is < 0.1M |
Solubility Product Constant
If a saturated aqueous solution of barium sulphate is taken, the following equation represents the equilibrium between the undissolved solids and ions: The equilibrium constant in the above case is: The concentration of pure solid substances remains constant, so we can say: In this case, Ksp is the solubility product constant, which means that, when solid barium sulphate is in equilibrium with its saturated solution, the product of both barium and sulphate ions is equal to Ksp.Also Check - Aluminium Nitrate Formula
Difference Between Solubility and Solubility Product Formula
Solubility and solubility product formulas are related concepts in chemistry, but they refer to different aspects of a substance's ability to dissolve in a solvent. Solubility : Definition: Solubility measures the maximum amount of a solute (usually a solid) that can dissolve in a specific amount of solvent at a given temperature and pressure to form a saturated solution. Units: Solubility is typically expressed in grams of solute per 100 milliliters (g/100 mL) or in moles per liter (mol/L) of solvent. Factors Affecting Solubility: Solubility is influenced by temperature, pressure, and the solute's and solvent's nature. Generally, some substances become more soluble with increasing temperature, while others become less soluble. Solubility Product Formula: Definition: The solubility product (Ksp) is an equilibrium constant that describes the degree to which an ionic compound dissolves in water to form its constituent ions. It is used specifically for sparingly soluble salts that do not fully dissociate in solution. Formula: The solubility product constant, Ksp, is calculated using the following general equation: For a salt with the chemical formula AaBb, its solubility product expression is Ksp = [A^a+][B^b-], where [A^a+] and [B^b-] are the concentrations of the ions A^a+ and B^b- in the saturated solution. Units: Ksp values are unitless because they are based on concentration ratios.Solubility Product Formula FAQs
What is the solubility product constant (Ksp)?
Ksp represents the equilibrium concentration product of ions in a sparingly soluble salt solution.
What is the difference between Ksp and solubility?
Solubility measures the maximum amount of a solute that can dissolve, while Ksp measures the extent of ion dissociation in a saturated solution.
When Qsp exceeds Ksp, what happens?
As a result of precipitation, a solid is formed in the solution.
Can Ksp values be used to predict a compound's solubility?
Yes, Ksp can be used to determine whether a solution is saturated or will precipitate by comparing it to the ion product (Qsp).
What is the effect of temperature on Ksp values?
For most salts, Ksp values increase with temperature, indicating a higher solubility at higher temperatures.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









