
Ammonium Hydroxide Formula: Ammonia solution, known by various names like ammonia water, ammonium hydroxide, and more, is a water-based solution of ammonia, often symbolized as NH 3 (aq). Despite its name suggesting an alkali with the formula [NH 4 ][OH], obtaining pure NH 4 OH samples is practically unfeasible since NH4+ and OH- ions make up a minimal portion of ammonia content, except in highly dilute solutions.
Ammonium is a positively charged ion resulting from the addition of protons to ammonia. Its chemical symbol is NH 4 + , and it is commonly referred to as an ammonia solution when dissolved in water. This solution goes by several other names, including ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, and sometimes inaccurately as ammonia. In chemical notation, it is typically denoted as NH 3 (aq).
Ammonium Hydroxide Formula
The chemical formula of ammonium hydroxide is NH 4 OH. Despite its name suggesting an alkali with the composition [NH 4 + ][OH – ], it is practically impossible to isolate pure NH 4 OH samples. In anything but extremely dilute solutions, the NH 4 + and OH – ions make up only a negligible portion of the total ammonia content.Ammonium Hydroxide Formula Structure
Ammonium Hydroxide Formula is NH 4 OH. The structure of ammonium hydroxide involves an ionic bond between a single hydroxide anion (OH – ) and one ammonium cation (NH 4 + ).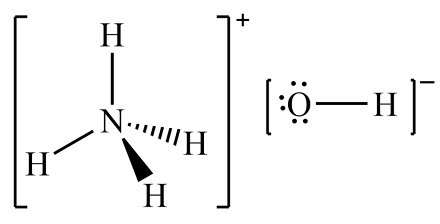
Ammonium Hydroxide Formula Physical Properties
Ammonium Hydroxide Formula is NH 4 OH.Ammonium hydroxide is a colorless liquid.
It has a distinct unpleasant fishy odor.
It readily mixes with water, making it water-soluble.
In water, it is miscible.
The specific gravity of a solution typically ranges around 0.9 but can vary based on the solution's strength.
The auto-ignition temperature is 651°C.
Its molecular weight is 35.05 g/mol.
Its density is 0.91 g/cm3.
At 20°C, the vapor pressure is 115 mm.
The melting point of NH4OH is -57.5°C.
The boiling point varies between 38°C and 100°C, depending on the concentration.
Ammonium Hydroxide Formula Chemical Properties
The basicity of ammonia in water is an important concept to understand. Ammonium hydroxide is considered a weak base, and when it's dissolved in water. it doesn't completely dissociate. Instead, it establishes an equilibrium with the formation of ammonium ions and hydroxide ions: NH 4 OH + H 2 O ⇌ NH 4 + + OH −Preparation of Ammonium Hydroxide
This equilibrium is significant because it allows us to control the pH in solutions. The presence of hydroxide ions (OH - ) in the solution raises the pOH and indicates its basicity level. So, ammonium hydroxide plays a role in influencing the pH and basicity of solutions.
To make Ammonium Hydroxide, you can create a simple chemical reaction by saturating water with gaseous ammonia. This process is represented by the equation:
NH 3 + H 2 O → NH 4 OH
Uses of Ammonium Hydroxide
Aqueous ammonia, unlike its anhydrous counterpart with limited applications, actually has quite a few practical uses:
Household Cleaning: You will find diluted aqueous ammonia (usually at 1-3% concentration) as a vital ingredient in various household cleaning products, particularly in window cleaning solutions. The cool part is that as the water evaporates during cleaning, the ammonia gas in it also evaporates, leaving you with streak-free, sparkling windows.
Standalone Cleaner: Sometimes, you can get plain aqueous ammonia as a standalone cleaner. It might also come in scented versions, like lemon (usually yellow) or pine (green). And when it's mixed with soap, it's often called "cloudy ammonia."
Absorption Refrigeration: Back in the early 1900s, people used water-ammonia systems for vapor absorption cooling. It was pretty popular until the vapor compression refrigeration system came along, boasting a much better performance. The Electrolux refrigerator and the Einstein refrigerator are famous examples of ammonia-based cooling systems.
Water Treatment: Aqueous ammonia is handy for producing chloramine, which is a disinfectant used in water treatment. It's favored over direct chlorination for drinking water because it remains effective in stagnant pipes, reducing the risk of waterborne infections.
Fish Tank Setup: If you are an aquarium enthusiast, you might use ammonia in the form of aqueous ammonia for a process called fishless cycling. The key here is that the ammonia must be free of any additives to ensure a healthy aquatic environment.
| Related Links | |
| Strontium Chloride Formula | Barium Bromide Formula |
| Barium Fluoride Formula | Hydrobromic Acid Formula |
Ammonium Hydroxide Formula FAQs
What is the primary purpose of ammonium hydroxide?
Is NH4OH an acid or a base?
What is the pH of NH4OH?
What is the color of NH4OH?










