
Barium Fluoride Formula: Barium fluoride, represented by the formula BaF 2 . It is a colorless solid with a natural occurrence in the form of the rare mineral known as frankdicksonite. Under standard conditions, it takes on the fluorite structure, but it can transition to the PbCl 2 structure under high-pressure conditions. Similar to calcium fluoride (CaF 2 ), it demonstrates resilience and remains insoluble in water.
At temperatures around 500°C and above, Barium fluoride is susceptible to corrosion when exposed to moisture. However, in dry environments, it can be safely used at temperatures as high as 800°C. Prolonged exposure to moisture can adversely affect its transmission capacity in the vacuum ultraviolet (UV) range. While it may be less resistant to water compared to calcium fluoride.
It stands out as the most resistant optical fluoride when it comes to withstanding high-energy radiation, even though its transmittance in the far-ultraviolet range is comparatively lower. Furthermore, it possesses a high level of hardness, but it is notably sensitive to thermal shock and can fracture relatively easily.
Barium, denoted as 'Ba' on the periodic table, is classified as a group 2 element with an atomic number of 56. This heavy alkaline earth metal is present in mineral deposits and comprises about 0.05 percent of the Earth's crust. Barium, along with its derivatives, can either occur naturally or be synthesized for various purposes. It hold a silvery-white appearance and can be naturally encountered, often in combination with other substances such as carbon, oxygen, and sulfur. Notably, its density is remarkably low, approximately half that of iron.
Fluoride refers to chemical compounds containing fluorine, often combined with various elements or groups, particularly salts featuring the F– anion, or organic compounds where fluorine is linked to an alkyl group. Fluoride is commonly used to prevent tooth decay as it can be absorbed by teeth, bolstering their strength, providing resistance against acids, and inhibiting the formation of cavities caused by bacteria.
Barium Fluoride Formula
Barium Fluoride Formula is BaF 2 . It is an inorganic chemical compound. This substance exists as a colorless solid and can be naturally found in the rare mineral known as frankdicksonite. It exhibits a fluorite structure but can adopt a PbCl 2 structure under high pressure conditions. When placed in water, it remains insoluble, similar to CaF 2 . Barium fluoride is susceptible to corrosion at temperatures exceeding 500 °C due to moisture. However, it can be safely used at temperatures of up to 800 °C in dry environments.Extended exposure to moisture can degrade the transmission capability of materials in the vacuum ultraviolet (UV) spectrum. While barium fluoride is relatively less resistant to water than calcium fluoride, it stands out as the most resilient among all optical fluorides when it comes to withstanding high-energy radiation, even though its transmittance in the far-ultraviolet range is lower. It's worth noting that barium fluoride is extremely hard, but it's susceptible to temperature shocks and tends to fracture easily.
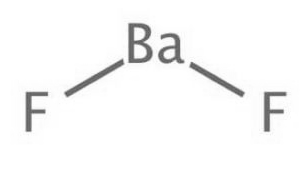
Barium Fluoride Formula Structure
Barium Fluoride Formula is BaF2. The chemical compound barium fluoride is denoted by the molecular formula BaF2. We can represent its structure visually through the typical organic molecule format.
Barium Fluoride Preparation
Barium Fluoride Formula is BaF 2 . In the laboratory, barium fluoride is typically produced when a positively charged barium atom reacts with two negatively charged fluoride atoms. This reaction can be described as follows:
Ba + + 2 F – → BaF 2
Barium Fluoride Formula Physical Properties
Barium Fluoride Formula is BaF 2. Barium fluoride exhibits several distinctive physical characteristics:
It forms white cubic crystals.
The refractive index of this compound ranges around 1.46 within the wavelength span of 700 nm to 5 µm.
Barium fluoride has high melting and boiling points, specifically 1368°C and 2260°C, respectively.
It holds a density of 4.89 g/cm³ and a molar mass of 175.34 g/mol.
It should be noted that prolonged exposure to moisture can reduce its transmission in the UV range.
In the presence of moisture, Barium fluoride is susceptible to corrosion; however, it can withstand temperatures up to 800°C in a dry environment.
Barium Fluoride Formula Chemical Properties
Barium Fluoride Formula is BaF 2. Barium fluoride displays several notable chemical reactions:
Double Displacement Reaction with Sodium Nitrate:
When one mole of barium fluoride interacts with two moles of aqueous sodium nitrate, it gives rise to one mole of nitrobarite and two moles of aqueous sodium fluoride, representing a double displacement reaction:
BaF 2 + 2NaNO 3 → Ba(NO 3 ) 2 + 2NaF Displacement Reaction with Zinc: When one mole of barium fluoride reacts with the element zinc, it results in the formation of barium and zinc fluoride through a displacement reaction: BaF 2 + 2Zn → Ba + 2ZnF Displacement Reaction with Diiodine: The reaction of one mole of barium fluoride with diiodine leads to the creation of barium iodide and fluorine, constituting a displacement reaction: BaF 2 + I 2 → BaI 2 + 2FBarium Fluoride Optical Properties
Barium fluoride (BaF 2 ) exhibits distinct optical properties:
It serves as a rapid and versatile scanner and widely used for the detection of X-rays, gamma rays, or high-energy particles.
Unlike most light-emitting substances, barium fluoride possesses the unique attribute of not emitting ultraviolet light.
When observed under ultraviolet light, it exhibits transparency within the infrared spectrum spanning from 150 to 200 nanometers and 11 to 11.5 micrometres.
Additionally, it can be utilized to detect high-energy neutrons through pulse shape discrimination.
Barium Fluoride Gas-Phase Molecular Structure
Barium Fluoride Formula is BaF 2 . In the gaseous phase, the BaF 2 molecule exhibits a non-linear configuration, with the F-Ba-F angle measuring approximately 108°. This non-linear arrangement deviates from the principles of the Valence Shell Electron Pair Repulsion (VSEPR) theory.
One explanation for this non-linearity is attributed to the influence of the (D) orbital from the electron shell located beneath the valence shell. Another hypothesis suggests that the electron nucleus polarization of the barium atom leads to a nearly tetrahedral distribution of charges, influencing the nature of the Ba-F bond.
Uses of Barium Fluoride
Barium fluoride finds diverse applications in various industries:
It plays a role in enamel production, contributing to the creation of durable, glossy coatings.
It is mainly used in the production of windows for infrared spectroscopy, facilitating precise analysis in this field.
In the filed of fuel oil analysis, barium fluoride plays a crucial role, aiding in the examination and assessment of fuel quality.
Barium fluoride is a key component in solder manufacturing, utilized in soldering iron coatings and solder powders.
This compound is extensively used in the manufacturing of optical instruments, enhancing the performance and capabilities of such devices.
Barium Fluoride Formula FAQs
What is the chemical formula for Barium Fluoride?
What is the structure of Barium Fluoride at standard conditions?
Is Barium Fluoride soluble in water?
In which applications is Barium Fluoride widely used?










