
Barium Bromide Formula: Barium bromide is a chemical compound represented by the formula BaBr 2 . This compound exhibits ionic characteristics and is known for its hygroscopic nature.
Barium, symbolized as 'Ba' on the periodic table, is a member of the group 2 elements with an atomic number of 56.
Barium is a dense alkaline earth metal that occurs naturally in ore deposits, accounting for roughly 0.05 percent of the Earth's crust. It is naturally present in the environment and can be manufactured for a wide variety of uses.
When exposed to oxygen then barium easily oxidizes and forms hydroxides and releases hydrogen in the process. Additionally, it exhibits a propensity to interact with virtually all non-metal elements, giving rise to the production of toxic substances.
Bromide refers to a compound of bromine that incorporates another element or group, such as a salt containing the Br– anion or an organic molecule where bromine is bonded to an alkyl radical.
Barium Bromide Formula
Barium Bromide Formula is BaBr 2 , stands as a notable chemical compound. It is also recognized as barium (2+) dibromide or anhydrous barium bromide. This substance presents itself in the form of a white solid that is soluble in water but proves toxic when in its aqueous state.
The crystalline structure of barium (2+) dibromide consists of white, deliquescent orthorhombic crystals, which resemble the patterns of lead chloride. In its aqueous solution, it behaves like a typical salt. When it reacts with the sulfate ion from sulfuric acid (H 2 SO 4 ), it produce barium sulfate (BaSO 4 ) precipitates, as depicted in the following chemical equation:
BaBr 2 (aq) + SO 4 2− → BaSO 4 (s) + 2 Br − (aq)
Barium Bromide Formula Structure
Barium Bromide Formula is BaBr 2 . Barium bromide is characterized by its precise mass, with a molecular weight of 297.74 g/mol and a monoisotopic mass of 297.742 g/mol. It does not act as a hydrogen bond donor, but it readily accepts two hydrogen bonds. The molecule comprises three covalently bonded units and is commonly canonicalized for its structural representation.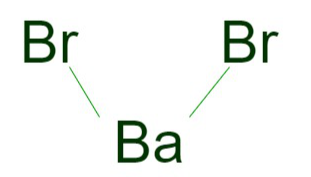
Barium Bromide Formula Physical Properties
Molecular Weight: Barium bromide possesses a molecular mass of 297.14 grams per mole, reflecting its atomic composition.
Density: The formula for barium bromide, BaBr2, is associated with a density of 4.78 g/cm3, denoting its relative heaviness.
Solubility: In its hydrated form, barium bromide readily dissolves in water at 75 degrees Celsius. Typically, it dissolves before the anhydrate phase decomposes, highlighting its solubility characteristics under specific conditions.
Melting Point: This inorganic substance exhibits a notably high melting point, reaching 857 degrees Celsius before it transforms from a solid to a liquid state.
Boiling Point: Barium bromide also boasts a substantial boiling point, which stands at 1835 degrees Celsius, indicating its stability at elevated temperatures.
Barium Bromide Formula Chemical Properties
Barium Bromide Formula is BaBr 2 . Barium bromide manifests as a white crystalline powder, consisting of three covalently linked BaBr2 units. Its molecular mass is 297.74 grams per mole, while the monoisotopic mass equals 297.742 grams per mole.
Within the composition of barium bromide, there are no hydrogen bond donors, and it features two hydrogen bond acceptors. This inorganic compound exhibits the following reactions:
Barium bromide reacts with sulfate salts to produce solid barium sulfate precipitate:
BaBr 2 + SO 4 2- → BaSO 4 + 2Br –
Interacting with oxalic acid leads to the formation of solid barium oxalate precipitates:
BaBr 2 + C 2 O 4 2- → BaC 2 O 4 + 2Br –
Barium bromide itself is an odorless white powder. Furthermore, when it comes into contact with hydrofluoric acid, it yields a solid barium fluoride precipitate:
BaBr 2 + F – → BaF 2 + 2Br –
Barium fluoride (BaF 2 ) is a colorless solid that can be naturally found.
Preparation of Barium Bromide
The production of Barium bromide involves a reaction between either barium carbonate or barium sulfide and hydrobromic acid (HBr) to yield hydrated barium bromide.
For barium sulfide (BaS):
BaS + 2HBr → BaBr 2 + H 2 S
For barium carbonate (BaCO 3 ):
BaCO 3 + 2HBr → BaBr 2 + CO 2 + H 2 O
Uses of Barium Bromide
Barium bromide (BaBr2) finds utility in numerous applications:
Chemicals utilize barium bromide as a precursor in various processes.
Historically, it was used in the purification of radium through a technique called fractional crystallization.
It plays a role in formation of other bromide compounds.
It plays a role in the field of photography.
Health Risks of Barium Bromide
The utilization of barium bromide carries several health hazards. Barium, as an inorganic substance, can impede the entry of intracellular potassium into cells, leading to the transfer of potassium from the extracellular to intracellular compartments. This reduction in resting membrane potential can render muscle fibers electrically unexcitable, potentially resulting in paralysis.
When barium bromide is ingested and enters the body, it poses a significant risk of poisoning.
Bromine, known for its strong oxidizing properties. It can release free oxygen radicals present in mucous membranes when in contact with water. These liberated radicals can function as strong oxidizers, causing damage to bodily tissues.
| Related Links | |
| Sodium peroxide Formula | Bromic Acid Formula |
| Sodium Nitride Formula | Cadmium Sulphate Formula |
Barium Bromide Formula FAQs










