
Hydrobromic Acid Formula is HBr. Hydrogen (H) holds a unique set of characteristics that makes it different from all other elements on Earth. Around two-thirds of the mass in our Universe is composed of this unique element. Hydrogen exhibits dual characteristics, being both electropositive and electronegative. This duality is evident in the formation of hydrogen cations (H+) and hydride anions (H–). Hydrogen compounds play a pivotal role as primary oxidants for various chemical substances present in the atmosphere, influencing the behavior of numerous chemical families.
Hydrogen finds application in the synthesis of ammonia (NH3). Bromine, with the chemical symbol Br, holds a significant position in the periodic table. The compound Br2, known as bromine, appears as a reddish-brown liquid with organic properties. It is rarely encountered in its pure form but is typically found in inorganic compounds known as bromides and organo-bromine compounds. These compounds are commonly present in soils, salts, the atmosphere, and seawater.
Hydrobromic Acid Formula
Hydrobromic acid formula is HBr. It is referred to as hydrogen bromide and chemically represented by HBr. It is a diatomic molecule composed of hydrogen and bromine atoms. This compound builds a covalent bond between hydrogen and bromine. It ranks as a strong acid due to the ease with which the covalent bond between its components can ionize, Due to the high electronegativity of the bromine atom. In fact, it is stronger than the hydrochloric acid in terms of acidity. Its molecular formula is simply HBr.
Hydrobromic acid forms when one molecule of hydrogen combines with one molecule of bromine gas, producing two molecules of this compound.
The chemical equation for the formation of hydrobromic acid, HBr, is as follows:
H 2 + Br 2 → 2HBrHydrobromic Acid Formula Structure
Hydrobromic Acid Formula is HBr. Hydrobromic acid is a diatomic molecule characterized by a single covalent bond connecting its bromine and hydrogen atoms. This bond Easily ionizes due to the high electronegativity of the bromine atom, resulting in the release of H+ ions. This property makes HBr a highly strong acid.
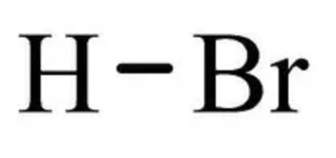
Preparation of Hydrobromic Acid
There are two primary methods for preparing hydrobromic acid:
Reaction with Bromine, Sulfur Dioxide, and Water:
This method involves the reaction of bromine with sulfur dioxide and water. formation of sulfuric acid as a by-product. The chemical equation for this process is as follows:
Br 2 + SO 2 + 2 H 2 O → H 2 SO 4 + 2 HBr
Reaction of Dilute Sulfuric Acid with Potassium Bromide:
Hydrobromic acid can also be prepared by reacting dilute sulfuric acid with potassium bromide. The chemical equation for this approach is as follows:
H 2 SO 4 + KBr → KHSO 4 + HBr
Hydrobromic Acid Formula Physical Properties
Hydrobromic acid exhibits various physical properties:
Hydrobromic Acid Formula is HBr.Molecular Weight: Hydrobromic acid has a molecular weight of 80.91 g/mol.
Density: Its density is 1.49 g/cm³.
Boiling Point: The boiling point of hydrobromic acid is 122 °C.
Melting Point: Its melting point is −11 °C.
Anhydrous Form: In its anhydrous state, it appears as a colorless gas with a pungent odor.
Aqueous Form: In its aqueous form, it is a colorless to faint yellow liquid with an acrid odor.
Corrosiveness: Hydrobromic acid is highly corrosive and ranks as a strong mineral acid. It is obtained by dissolving the diatomic molecule, hydrogen bromide, in water.
pKa Value: The pKa value of HBr acid is -9. It is a colorless or very pale yellow liquid.
Hydrobromic Acid Formula Chemical Properties
Hydrobromic acid displays distinct chemical properties:
Acidity: It is an exceptionally strong acid, surpassing even hydrochloric acid in strength.
Reaction with Bases: When it encounters bases, it forms bromide salts.
Reactivity with Metals: Hydrobromic acid is highly reactive and corrosive when in contact with most metals.
Reaction with Sulfuric Acid: When hydrobromic acid reacts with sulfuric acid, it produces sulfur dioxide, bromine, and water. The chemical equation for this reaction is as follows:
2HBr + H 2 SO 4 → Br 2 + SO 2 + 2H 2 O
Reaction with Propene: In the presence of propene, hydrobromic acid yields 2-Bromopropane. The reaction is represented as:
HBr + C 3 H 6 → C 3 H 7 Br
Uses of Hydrobromic Acid
Hydrobromic acid finds several practical uses:
It plays an important role in industrial for synthesizing a range of valuable inorganic bromides and organobromine compounds, including substances like zinc bromide, allyl bromide, and bromoacetic acid.
This acid is used in the extraction of specific metal ores, contributing to various metallurgical processes.
Hydrobromic acid serves as a common reagent in organic chemistry, facilitating oxidation and catalytic processes.
| Related Links | |
| Hydrogen Sulfate Formula | Barium Sulphate Formula |
| Bismuth(III) Chloride Formula | Borax Formula |
Hydrobromic Acid Formula FAQs
What is the formula for Hydrobromic Acid?
How is Hydrobromic Acid formed?
What are the key physical properties of Hydrobromic Acid?
What are the chemical properties of Hydrobromic Acid?
How is Hydrobromic Acid prepared?










