
Average Speed of Gas Molecules: Understanding the behaviour of gas molecules has been a basic of scientific inquiry, particularly in the field of chemistry. The kinetic theory of gases elucidates how gas molecules move and interact with each other. One critical aspect of this theory is the concept of molecular speed, which quantifies the average speed of a group of gas molecules. By discussing the formulas for the calculation of average molecular speed, we gain deeper insights into the dynamic nature of gases and their behaviors.
The Concept of Molecular Speed
The kinetic theory of gases postulates that gas molecules are in a state of non stop motion. They travel in straight lines until they collide with other molecules, exhibiting properties of ideal behaviour such as constant and random motion. This movement and collision process is governed by various measures of molecular speed, which are fundamental in comprehending the behaviour of gases in different conditions.
Average Speed of Gas Molecules
The average speed of gas molecules (Cavg ) is a vital parameter in understanding gas behaviour. It can be calculated using a formula derived from kinetic theory:
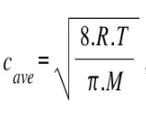
Where:
Cavg represents the average speed of the gas molecules.
R is the ideal gas constant (8.314 kgm²/s²mol*K).
T stands for the temperature of the gas in Kelvin.
M denotes the molar mass of the gas.
Temperature (T): The temperature of the gas directly influences the average speed of gas molecules. As temperature rises, the kinetic energy of gas molecules increases, leading to higher average molecular speed. Consequently, lower temperatures result in decreased molecular speed.
Molar Mass (M): The molar mass of a gas is inversely proportional to its average molecular speed. Lighter gases exhibit higher molecular speeds compared to heavier ones. For instance, helium, with the lowest molar mass among common gases, demonstrates a higher average molecular speed.
Implications and Significance
Diffusion and Effusion: The concept of molecular speed and its calculation are crucial in understanding diffusion and effusion phenomena. Lighter gas molecules diffuse more rapidly than heavier ones due to their higher average speeds.
Gas Behavior and Properties: The average speed of gas molecules plays a pivotal role in determining various properties of gases, such as pressure, temperature, and volume.
Real-life Applications: Understanding the average molecular speed of gases is fundamental in fields like environmental science, engineering, and industrial applications, where gas behaviors need to be predicted and controlled.
The formula for calculating the average speed of gas molecules serves as a fundamental tool in the study of gas behaviors and properties. It enables scientists and researchers to comprehend and predict gas-related phenomena in diverse fields. This knowledge has wide-ranging applications in industries and scientific research, allowing for innovations and advancements that shape our modern world.
The exploration and understanding of the average speed of gas molecules continue to be a cornerstone in the field of chemistry, unraveling the mysteries of the behavior of gases and their significant impacts on various scientific and industrial domains.
Applications of Average Speed of Gas Molecules
Scientific Research: Essential for studying gas properties and behaviors.
Industrial Applications: Vital in optimizing manufacturing processes and engineering applications.
Environmental Science: Crucial for managing air quality and predicting environmental impacts.
Technology Development: Important in designing gas sensors, reactors, and fuel cells.
Medical and Pharmaceutical Research: Helps in drug development and understanding gas behavior in biological systems.
Astronomy and Space Science: Key for interpreting planetary atmospheres and space propulsion systems.
Gas Separation and Purification: Aids in designing processes for gas separation and purification.
Energy and Power Generation: Essential for optimizing energy conversion processes.
The formula's applications span scientific, industrial, environmental, medical, and technological domains, impacting research, development, and practical implementations across various sectors.
PW Neev Fastrack and Udaan Fastrack Batch are Specially designed for class 9 and 10 students. Enrol Now Online Course of Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your chemistry knowledge. and build a strong foundation.
| Related Links | |
| Pyrophosphoric Acid Formula | Potassium Sulfite Formula |
| Potassium Permanganate Formula | Dinitrogen Pentoxide Formula |
Average Speed of Gas Molecules FAQs
How does temperature affect the average speed of gas molecules?
What is the influence of molar mass on molecular speed?
Why is understanding molecular speed important?
What are the practical applications of knowing the average speed of gas molecules?










