
Pyrophosphoric Acid Formula: Hydrogen is an elemental substance denoted by the chemical symbol H and possesses an atomic number of 1. It exhibits qualities such as being transparent, lacking a discernible odor or taste, while being non-harmful and flammable. Hydrogen primarily exists in a gaseous state at standard conditions and holds the distinction of being the most prevalent element in the cosmos. Each hydrogen atom consists of a solitary proton, making it an ideal choice as a clean and versatile fuel source suitable for a wide array of applications.
Phosphorus, on the other hand, is a chemical element symbolized by P and characterized by an atomic number of 15, with an electronic configuration of 1s² 2s² 2p⁶ 3s² 3p³. It is found in two primary forms, namely white phosphorus and red phosphorus. Phosphorus is abundantly distributed in nature and holds significance as an essential mineral. It occurs naturally in various foods and is also available as a dietary supplement.
Oxygen, represented by the chemical symbol O and having an atomic number of 8, possesses an electronic configuration of 1s² 2s² 2p⁴. It belongs to the chalcogen group on the periodic table. Oxygen is a colorless, odorless, and tasteless gas vital to the existence of living organisms. It stands as the most abundant element in the Earth's crust and is recognized as a highly reactive element, being present in water, the majority of rocks and minerals, as well as numerous organic compounds.
Pyrophosphoric Acid Formula
Pyrophosphoric acid, also known as diphosphoric acid, is an inorganic compound characterized by the chemical formula H 4 P 2 O 7 . This compound is colorless and lacks a discernible odor, while being soluble in water, ethyl alcohol, and diethyl ether. It serves as an anhydride of acrylic phosphorus acid and qualifies as a chemical substance. Pyrophosphoric acid finds utility as a catalyst and plays a role in organic chemical manufacturing. It is produced through the combination of one molecule of phosphorus pentoxide with two molecules of water, with its invention credited to Mr. Clarke of Glasgow in 1827.
Pyrophosphoric Acid Formula Structure
Pyrophosphoric Acid is an acyclic phosphorus acid anhydride that results from the condensation of two molecules of phosphoric acid. The structural representation of Pyrophosphoric Acid is illustrated in the following diagram:
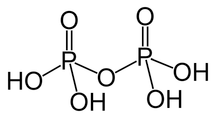
In this structure, it is evident that two phosphate groups interact with four hydroxyl groups, capable of donating protons when dissolved in an aqueous solution.
Production of Pyrophosphoric Acid Preparation
Pyrophosphoric Acid is synthesized from phosphoric acid through various methods:
Direct Reaction:
2 H 3 PO 4 → H 2 O + H 4 P 2 O 7
Using Phosphorus Pentoxide:
2 H 2 O + P 2 O 5 → H 4 P 2 O 7
Involving Phosphorus Acid and Phosphorus Pentoxide:
4 H 3 PO 4 + P 2 O 5 → 3 H 4 P 2 O 7
Additionally, Pyrophosphoric Acid can be obtained by reacting phosphoric acid with phosphoryl chloride:
5 H 3 PO 4 + POCl 3 → 3 H 4 P 2 O 7 + 3 HCl
Pyrophosphoric Acid Formula Physical Properties
- Pyrophosphoric Acid Formula: Pyrophosphoric Acid Formula is H 4 P 2 O 7
- Molecular Weight: 177.973 g/mol
- Chemical Names: Diphosphoric acid, Pyrophosphoric acid, 2466-09-3 Phosphonooxyphosphonic acid, acide pyrophosphorique
- Solubility in Water: It is soluble in water.
- Melting Point: Its melting point is 71.5°C.
- Conjugated Base: Pyrophosphate
Pyrophosphoric Acid Formula Chemical Properties
When Pyrophosphoric Acid interacts with sodium hydroxide, it generates water and trisodium diphosphate as follows:
3 NaOH + H 4 P 2 O 7 → 3 H 2 O + Na 3 HP 2 O 7
In the presence of water, Pyrophosphoric Acid transforms into phosphoric acid:
H 2 O + H 4 P 2 O 7 → 2 H 3 PO 4
Combining Pyrophosphoric Acid with sodium hydroxide results in the formation of tetrasodium diphosphate and water:
3 NaOH + H 4 P 2 O 7 → 3 H 2 O + Na 3 HP 2 O 7
Uses of Pyrophosphoric Acid
Pyrophosphoric acid finds utility as an electrolyte within fuel cells.
It serves as an etching solution in various industrial processes.
It is utilized as an ingredient for preventing iron deficiency in certain applications.
It is harnessed as a biochemical energy source in specific contexts.
Pyrophosphoric acid is used as a catalyst in chemical reactions.
| Related Links | |
| Potassium Bromate Formula | Potassium Chlorate Formula |
| Potassium Hexacyanoferrate (III) Formula | Potassium Fluoride Formula |
Pyrophosphoric Acid Formula FAQs
What is the chemical formula for pyrophosphoric acid?
How is pyrophosphoric acid produced?
What are the physical properties of pyrophosphoric acid?
What are the chemical properties of pyrophosphoric acid?










