
Potassium Sulfite Formula : Potassium, represented by the symbol K, is an essential chemical element vital for the proper functioning of all bodily tissues. It plays a crucial role in maintaining the normal operation of cells. Its primary application is in the production of potassium chloride, a key ingredient in fertilizer manufacturing, thus contributing significantly to plant growth. Potassium stands as the most abundant intracellular cation and is indispensable as a dietary nutrient.
Sulfur, denoted by the symbol S, belongs to the nonmetallic elements within the oxygen group. Its main applications encompass the production of sulfuric acid and the vulcanization of black rubber. Sulfur can be found both in its native form and within metal sulfide ores. This versatile element is utilized in the creation of car batteries, as well as in the fields of fertilizer production, water treatment, and mineral extraction.
Oxygen, with the symbol O, exists as a colorless, odorless, and tasteless gas, constituting an essential component of the air we breathe. This element is indispensable for sustaining life and finds extensive usage in various industries, water treatment processes, and medical therapy. Inadequate oxygen circulation within the bloodstream can impede the heart's ability to effectively pump blood to the body's tissues.
Potassium Sulfite Formula
Potassium sulfite, also known as dipotassium sulfite, is an inorganic, non-flammable compound with the Potassium sulfite formula is K 2 SO 3 . This substance is a white, soluble salt formed from the potassium cation and the sulfite anion. Its primary application lies in the food industry, where it serves as a preservative.Potassium Sulfite Formula and Charge
The potassium sulfite formula is K 2 SO 3 . In this formula:
"K" represents potassium, which has a charge of +1. This means each potassium ion (K + ) carries a single positive charge.
"SO 3 " represents the sulfite ion, which consists of one sulfur atom and three oxygen atoms. The overall charge of the sulfite ion is -2. Therefore, the Potassium sulfite is formula K 2 SO 3 indicates that there are two potassium ions (2x K + ) and one sulfite ion (SO 3 2- ) in the compound. This combination of ions balances the charges, resulting in a neutral compound.
Potassium Sulfite Formula Structure
T o derive the chemical formula for potassium sulfite, we begin by identifying the constituent elements. Potassium, denoted by the symbol K, belongs to group I of the periodic table and carries a +1 ionic charge, denoted as K +1 . The sulfite ion, SO 3 2- , comprises one sulfur atom and three oxygen atoms, possessing a total of 24 valence electrons.
The diagram shows the structure of potassium sulfite.
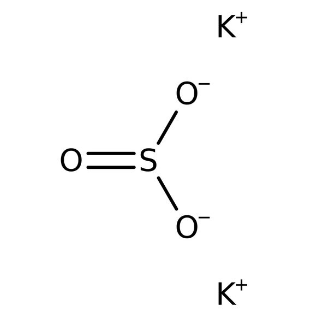
When drawing the Lewis structure for the sulfite ion, we observe that it consists of one sulfur atom and three oxygen atoms. Sulfur has 6 valence electrons, while each of the three oxygen atoms also has 6 valence electrons, amounting to a total of 18. In this arrangement, sulfur serves as the central atom due to its singular presence.
However, to achieve electron octets and satisfy the bonding requirements, one of the oxygen atoms shares one of its lone pairs with sulfur, forming a double bond between oxygen and sulfur. This arrangement ensures that both sulfur and oxygen possess 8 valence electrons, maintaining chemical stability.
Potassium Sulfite Preparation
Preparation of Potassium Sulfite involves several methods: Decomposition of Potassium Metabisulfite: Potassium Sulfite is obtained by breaking down potassium metabisulfite, resulting in the formation of potassium sulfite and sulfur dioxide (SO 2 ). K 2 S 2 O 5 → K 2 SO 3 + SO 2 Reaction of Sulfurous Acid with Potassium Hydroxide: When sulfurous acid (H 2 SO 3 ) reacts with potassium hydroxide (KOH), it produces potassium sulfite and water. H 2 SO 3 + 2 KOH → K 2 SO 3 + 2 H 2 O Production from Potassium Sulfate: Potassium sulfite can also be made from potassium sulfate (K 2 SO 4 ) by a chemical process that yields potassium sulfite and oxygen (O 2 ). 2 K 2 SO 4 → 2 K 2 SO 3 + O 2 Redox Reaction: A redox reaction involving potassium (K), sulfur (S), and oxygen (O) results in the formation of potassium sulfite. 2 K + S + 3 O → K 2 SO 3 Reaction with Manganese Sulfate and Potassium Manganate: By combining manganese sulfate with potassium manganate and water, potassium sulfite is produced along with manganese dioxide and sulfuric acid. 4 MnSO 4 + 2 KMnO 4 + 3 H 2 O → K 2 SO 3 + 6 MnO 2 + 3 H 2 SO 4Potassium Sulfite Formula Physical Properties
Physical Properties of Potassium Sulfite:
Molecular weight: 158.254 g/mol
Appearance: White powder
Density: 2.35 g/cm³
Melting point: Above 590°C
Potassium Sulfite Formula Chemical Properties
Chemical Properties of Potassium Sulfite: Chemical formula: Potassium Sulfite formula is K 2 SO 3 Reaction with sulfuric acid: Potassium Sulfite reacts with sulfuric acid to produce potassium bisulfate, water, and oxygen. K 2 SO 3 + 2 H 2 SO 4 → 2 KHSO 4 + H 2 O + O 2 Reaction with oxygen: Potassium Sulfite reacts with oxygen to form Potassium Sulfate. 2 K 2 SO 3 + O 2 → 2 K 2 SO 4 Reaction with hydrobromic acid: It reacts with hydrobromic acid to generate potassium bromide, sulfur dioxide, and water. 2 HBr + K 2 SO 3 → 2 KBr + SO 2 + H 2 O Reaction with hydrochloric acid: Potassium Sulfite reacts with hydrochloric acid to produce potassium chloride and sulfurous acid. K 2 SO 3 + 2 HCl → 2 KCl + H 2 SO 3 Reaction with nitric acid: When it reacts with nitric acid, it forms potassium sulfate, nitrogen dioxide, and water. K 2 SO 3 + 2 HNO 3 → K 2 SO 4 + 2 NO 2 + H 2 OHealth Hazards of Potassium Sulfite
It can cause eye and skin irritation.
May lead to gastric irritation.
Ingesting large doses may result in severe health consequences, including the possibility of death.
Safety Measures for Handling Potassium Sulfite:
Can cause respiratory irritation.
Potassium Sulfite Prevention
Carefully review all provided instructions before use.
Prevent contact with eyes and skin.
Avoid inhalation of fumes or dust.
Store the substance in its original containers, ensuring they are securely sealed.
Wear protective eyewear and gloves when working with it.
Minimize exposure to the environment, if possible.
Uses of Potassium Sulfite
Frequently used as a food preservative.
Applied across various industries.
Sometimes used to precipitate gold from Aqua Regia solutions.
Utilized as a reducing agent in the chemical sector.
Valuable for dyeing textiles and cotton printing in the textile industry.
| Related Links | |
| Potassium Chlorate Formula | Potassium Bromate Formula |
| Gold Formula | Phosphate Formula |
Potassium Sulfite Formula FAQs
What is the chemical formula for potassium sulfite?
What is the primary application of potassium sulfite?
How is potassium sulfite's chemical structure determined?
What are some methods for preparing potassium sulfite?
What are the physical properties of potassium sulfite?










