
Dinitrogen Pentoxide Formula : The formula for Dinitrogen Pentoxide consists of 2 nitrogen atoms and 5 oxygen atoms. Nitrogen, denoted by the symbol N, is a widely occurring chemical element with an atomic number of 7, and it is abundantly present in the Earth's crust. Classified as a nonmetal, nitrogen is the lightest member of Group 15 in the periodic table. Its applications encompass food preservation, packaging, as well as its use in the light bulb industry, chemical analysis, and various sectors within the chemical industry.
Oxygen, symbolized as O, is another chemical element with an atomic number of 8. It ranks among the most prevalent elements in the Earth's crust. Oxygen plays a crucial role in numerous combustion processes and serves as an oxidizing agent in rocket fuels. Furthermore, it finds utility in artificial respiration.
Dinitrogen Pentoxide Formula
Dinitrogen Pentoxide is a chemical compound composed solely of oxygen and nitrogen atoms. It takes on the anhydride form of nitric acid and can crystallize in a colorless state. This compound consists of two nonmetals, oxygen and nitrogen, and has a chemical formula of N 2 O 5 . When at room temperature, it decomposes into nitrogen oxide and NO 2 .
It is also commonly referred to as nitric anhydride and is recognized for its potent oxidizing properties. Dinitrogen Pentoxide is extensively utilized as a strong oxidizer in high-thrust rocket propulsion systems and holds significance as a key component in the universe.
Dinitrogen Pentoxide Formula Ionic or Covalent
Dinitrogen Pentoxide (N 2 O 5 ) primarily forms covalent bonds due to the sharing of electrons between nitrogen and oxygen atoms.Dinitrogen Pentoxide Formula Structure
The structural arrangement of Dinitrogen Pentoxide, denoted by the chemical formula N 2 O 5 , can be elucidated through a Lewis structure. To construct this structure, it is essential to determine the total number of valence electrons, which amounts to 40. These electrons are subsequently used in forming ionic bonds.
A practical approach to expeditiously sketch the structure is to initiate with a basic framework, designating oxygen as the central atom. The valence electrons are then assigned to each atom until their octets are satisfied. As a result, all the atoms, with the exception of the central oxygen, attain octet fulfillment, as it possesses only 4 valence electrons.
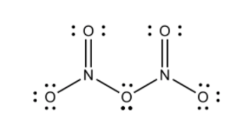
Dinitrogen Pentoxide Preparation
Dinitrogen Pentoxide can be prepared through various reactions:
Reaction with Phosphorus Pentoxide and Nitric Acid:
Dinitrogen Pentoxide is obtained when phosphorus pentoxide reacts with nitric acid as shown in the equation:
2 HNO 3 + P 2 O 5 → N 2 O 5 + 2 HPO 3
Oxygen and Nitrogen Reaction:
Dinitrogen Pentoxide can also be formed through the reaction of oxygen with nitrogen:
5 O 2 + 2 N 2 → 2 N 2 O 5
Reaction with Nitrogen Dioxide and Trioxygen:
Nitrogen dioxide reacts with trioxygen to produce Dinitrogen Pentoxide:
2 NO 2 + O 3 → O 2 + N 2 O 5
Reaction with Nitrogen Monoxide and Dioxygen:
Another method involves the reaction of nitrogen monoxide with dioxygen:
3 O 2 + 4 NO → 2 N 2 O 5
Dinitrogen Pentoxide Formula Physical Properties
Molecular weight: 108.01 g/mol
Density: 1.64 g/cm3
Melting point: 41°C
Boiling point: 47°C
Dinitrogen Pentoxide Formula Chemical Properties
Chemical formula: Dinitrogen Pentoxide Formula is N 2 O 5
Reaction with Sodium Hydroxide: Dinitrogen Pentoxide reacts with sodium hydroxide to form sodium nitrate and water.
2 NaOH + N 2 O 5 → H 2 O + 2 Na 2 NO 3
Reaction with Potassium Hydroxide: Dinitrogen Pentoxide reacts with potassium hydroxide to form Potassium Nitrate and water.
2KOH + N 2 O 5 → H 2 O + 2KNO 3
Reaction with Water: Dinitrogen reacts with water to form nitric acid.
N 2 O 5 + H 2 O → 2 HNO 3
Reaction with Oxygen: Dinitrogen pentoxide reacts with oxygen to form nitrogen dioxide.
2 N 2 O 5 → O 2 + 4 NO 2
Uses of Dinitrogen Pentoxide
Dinitrogen Pentoxide finds diverse applications, serving as:
A Nitrating Agent: It plays a crucial role in contemporary synthetic organic chemistry as a nitrating agent, facilitating various chemical reactions.
Solvent: It is used as a solvent for various compounds, aiding in the dissolution and manipulation of substances.
Reagent for Nitration: In the realm of chemical reactions, it is utilized as a reagent, often dissolved in chloroform, to facilitate nitration processes.
Explosive Production: It is an essential component in the formulation of explosives.
Oxidizer in Rocket Propulsion: Dinitrogen Pentoxide serves as a potent oxidizer in high-thrust rocket engines, contributing to their propulsive power.
| Related Links | |
| Potassium Chlorate Formula | Potassium Bromate Formula |
| Phosphate Formula | Gold Formula |
Dinitrogen Pentoxide Formula FAQs
What is the chemical formula of Dinitrogen Pentoxide?
What is the structure of Dinitrogen Pentoxide?
How is Dinitrogen Pentoxide prepared?
What are the physical properties of Dinitrogen Pentoxide?
What are the chemical properties of Dinitrogen Pentoxide?










