
Sodium Peroxide Formula: Sodium (Na) is classified as an alkali metal, featuring an atomic number of 11 on the periodic table. This highly reactive, soft, silvery-white metal contrasts with oxygen (O), which hold an atomic number of 8. Oxygen, in its role as a highly reactive nonmetal and oxidant, readily forms oxides with various elements and compounds.
Sodium peroxide, with the chemical formula Na₂O₂, is an inorganic compound. This solid substance, tinted yellow, is the result of igniting sodium in an oxygen-rich environment. It holds strong alkaline properties.
Sodium Peroxide Formula
Sodium Peroxide formula is Na 2 O 2. Sodium Peroxide, an inorganic chemical compound, results from the combination of sodium and oxygen. This compound exhibits a yellowish hue and strong basic properties. It goes by various names, including Disodium dioxide, Solozone, or Flocool. Sodium Peroxide can be found in hydrate and peroxhydrate forms, such as Na 2 O 2 ·2H 2 O 2 ·4H 2 O, Na 2 O 2 ·2H 2 O, Na 2 O 2 ·2H 2 O 2 , and Na 2 O 2 ·8H 2 O. Its chemical formula is Na 2 O 2 .Sodium Peroxide Structure
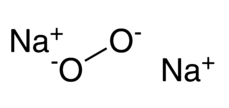
Sodium Peroxide formula is Na 2 O 2. Disodium dioxide appears as a granular solid with a color spectrum ranging from yellow-white to yellow. When it comes into contact with any combustible substance. it readily ignites when exposed to heat, moisture, or friction. Upon longer exposure to heat. it undergoes a highly energetic decomposition process. On a larger industrial scale. Disodium dioxide can be produced by subjecting metallic sodium to oxygen within a temperature range of 130 to 200 °C. Sodium Peroxide boasts a crystalline hexagonal structure.
Sodium Peroxide + Water
Sodium Peroxide formula is Na 2 O 2. When Sodium Peroxide is added to water. it undergoes a chemical reaction, yielding sodium hydroxide (NaOH) and hydrogen peroxide (H 2 O 2 ):
Na 2 O 2 + 2H 2 O → 2NaOH + H 2 O 2
This reaction results in the production of sodium hydroxide and hydrogen peroxide.
Preparation of Sodium Peroxide
Sodium Peroxide can be synthesized through several methods. One approach involves the reaction of sodium hydroxide with hydrogen peroxide:
2NaOH + H 2 O 2 → Na 2 O 2 + 2 H 2 O.
Another method entails the reaction of metallic sodium with oxygen at temperatures ranging from 130 to 200 °C. In the initial stage, sodium oxide is formed, and in a subsequent reaction:
4Na + O 2 → 2 Na 2 O
2Na 2 O + O 2 → 2 Na 2 O 2 .
Additionally, Sodium Peroxide can be obtained by passing ozone gas over solid sodium iodide inside a platinum or palladium tube, where the ozone serves to oxidize the sodium, ultimately yielding sodium peroxide.
Sodium Peroxide Formula Physical Properties
Sodium Peroxide has a molecular mass of 77.98 g/mol.
It exhibits a density of 2.805 g/cm3.
The melting point of Sodium Peroxide is 460 °C.
Its boiling point is 657 °C.
This compound appears as a yellow to white powder.
It demonstrates solubility in water, ethanol, and acids, while being insoluble in bases.
The magnetic susceptibility (χ) of Sodium Peroxide is measured at -28.10*10^-6 cm3/mol.
Sodium Peroxide Formula Chemical Properties
Chemical Properties of Sodium Peroxide:
When Sodium Peroxide is subjected to heat, its hexagonal structure undergoes a transition at 512 °C. Upon further heating beyond its boiling point of 657 °C, the compound decomposes into Na 2 O and releases oxygen:
2Na 2 O 2 → 2Na 2 O + O 2 .
In the presence of water. Sodium Peroxide reacts to produce sodium hydroxide and hydrogen peroxide through the following reaction:
Na 2 O 2 + 2H 2 O → 2NaOH + H 2 O 2 .
When Sodium Peroxide encounters carbon dioxide, it yields sodium carbonate and oxygen as a result of the following reaction:
2Na 2 O 2 + 2CO 2 → 2Na 2 CO 3 + O 2 .
Used of Sodium Peroxide
Sodium Peroxide finds utility in the bleaching of wood pulp.
It plays a important role in the manufacturing of paper and textiles.
As an effective oxidizing agent, Sodium Peroxide facilitates various chemical processes.
Its usefulness extends to applications in scuba gear and submarines.
It serves as an essential component in the extraction of minerals from diverse ores.
| Related Links | |
| Dinitrogen Pentoxide Formula | Potassium Nitrate Formula |
| Potassium Iodate Formula | Fluorine Gas Formula |
Sodium Peroxide Formula FAQs
What is the chemical formula of Sodium Peroxide?
What is the appearance of Sodium Peroxide?
What happens when Sodium Peroxide reacts with water?
What is the structure of Sodium Peroxide?
What are the main chemical properties of Sodium Peroxide?










