
Bromic Acid Formula: Bromic acid, alternatively referred to as hydrogen bromate, is classified as an oxoacid, and its chemical formula is HBrO 3 . Notably, it solely exists in an aqueous solution. This solution is transparent, but it undergoes a color change to yellow at room temperature as it decomposes into bromine. Bromic acid, along with bromates, exhibits potent oxidizing properties.
Hydrogen (H) Holds unique properties that distinguish it from all other elements found on Earth. It constitutes approximately two-thirds of the total mass in our Universe. Hydrogen demonstrates both electropositive and electronegative characteristics, giving rise to the formation of the hydrogen cation H+ and the hydride anion H-. Hydrogen compounds serve as crucial oxidizing agents for a variety of substances in the atmosphere, playing a significant role in the behavior of numerous chemical families. Notably, hydrogen is used in the production of ammonia (NH 3 ).
Bromine (Br) holds a prominent place in the periodic table and is known for its compound Br2, which exists as a reddish-brown liquid organic compound. It is rarely encountered in its pure elemental form, typically appearing in inorganic compounds referred to as bromides and organo-bromine compounds. These compounds are commonly found in soils, salts, the atmosphere, and seawater.
Oxygen is an odorless, colorless gas. It has atomic number of 8 on the periodic table. It plays an important role as a life-sustaining component of the atmosphere. Oxygen has up to 21% of Earth's air.
Bromic Acid Formula
Bromic Acid, with the chemical formula HBrO 3 , is also known as Hydrogen bromate or Bromic (V) acid. It exists exclusively in aqueous solutions, appearing initially colorless but turning yellow as it decomposes into bromine at room temperature. It is a potent oxidizing agent and can be synthesized by reacting barium bromate (Ba(BrO 3 ) 2 ) with sulfuric acid (H 2 SO 4 ). The resulting barium sulfate precipitate can be separated, leaving behind pure bromic acid.
Bromic Acid Formula
The chemical formula for Bromic acid is HBrO 3 . Bromic Acid, with the chemical formula HBrO3, is also known as Hydrogen bromate or Bromic (V) acid. It exists exclusively in aqueous solutions, appearing initially colorless but turning yellow as it decomposes into bromine at room temperature.Bromic Acid Formula Structure
Its molecular structure consists of one hydrogen (H) atom, one bromine (Br) atom, and three oxygen (O) atoms, arranged in a way that forms the Bromic acid molecule. Among its isomers, HBrO 3 is the most stable.
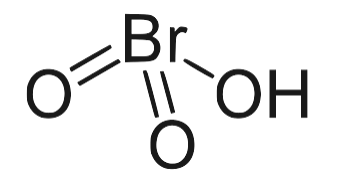
Bromic Acid Formula Physical Properties
Molecular weight: 128.91 g/mol
Conjugate base: Bromate
pKa: -2
Complexity: 46.2
Bromic Acid Formula Chemical Properties
Bromic acid can be produced by passing chlorine through bromine water using the following reaction:
5Cl 2 + Br 2 + 6H 2 O → 2HBrO 3 + 10HCl.
Bromic acid salts are stable at regular conditions, but upon heating, they decompose and release oxygen:
2KBrO 3 → 2KBr + 3O 2 .
Bromates are obtained through the electrochemical oxidation of the corresponding bromides. In acidic solutions, both bromic acid and its salts demonstrate strong oxidizing properties, reducing to the Br– ion or releasing free bromine.
Uses of Bromic Acid
Bromic acid finds various practical uses, including:
Bromate Production: Bromic acid serves as a key ingredient in the manufacturing of bromates, which are essential for producing inorganic bromides such as zinc, calcium, and sodium.
Ore Extraction: It plays a role in the extraction processes of certain ores, aiding in the separation and purification of valuable minerals.
Oxygen-Free Radical Generation: Bromic acid is capable of releasing oxygen-free radicals when in contact with water in mucous membranes, contributing to specific biochemical reactions and processes.
Organobromine Compound Synthesis: It can used as a reagent for the synthesis of organobromine compounds, a crucial class of organic molecules.
Alkylation Catalyst: Bromic acid acts as a catalyst for alkylation reactions, facilitating the introduction of alkyl groups into organic compounds.
| Related Links | |
| Pyrophosphoric Acid Formula | Potassium Sulfite Formula |
| Dinitrogen Pentoxide Formula | Potassium Nitrate Formula |
Bromic Acid Formula FAQs
What is the chemical formula of bromic acid?
What happens to the color of a bromic acid solution at room temperature?
What is the significance of bromic acid in chemistry?
How can you produce bromic acid?










