

A Biomolecule , also known as a biological molecule, is a molecule that is produced by living organisms and plays a crucial role in various biological processes. These molecules are typically organic compounds, which means they contain carbon atoms bonded to other atoms such as hydrogen, oxygen, nitrogen, and sometimes sulfur or phosphorus. Biomolecules are essential for the structure, function, and regulation of living organisms.
Carbohydrates are organic molecules composed of carbon, hydrogen, and oxygen. They primarily serve as energy storage and transport molecules in plants and animals but also have structural and signalling roles. Commonly recognized forms include sugars, starches, and celluloses.
Carbohydrates
Carbohydrates are typically classified based on their complexity: Monosaccharides: These are the simplest form of carbohydrates that cannot be hydrolyzed further to give simpler units. Examples include glucose, fructose, and galactose.
Disaccharides: These are composed of two monosaccharide units. Examples include sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (glucose + glucose).
Oligosaccharides: These consist of 3 to 10 monosaccharide units.
Polysaccharides: These are usually complex carbohydrates composed of more than ten monosaccharide units. Examples include starch, cellulose, and glycogen.
Glucose:
Preparation: One common method of preparing glucose is from starch by hydrolysis, first to produce maltose and then glucose:
Starch + H 2 O (in presence of acid) → 2 Glucose
(C 6 H 10 O 5 )n (Starch) + nH 2 O→ nC 6 H 12 O 6 (Glucose)
Monosaccharide 1+Monosaccharide 2 → Disaccharide + H 2 O
C 6 H 12 O 6 (Glucose) + C 6 H 12 O 6 (Fructose) → C 12 H 22 O 11 (Sucrose)+H 2 O
Structure:
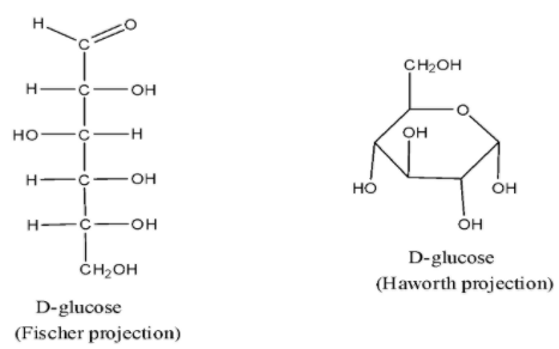
Glucose has a linear and a cyclic structure. The cyclic structure is more stable and exists predominantly in solutions. The cyclic form can exist in alpha (α) and beta (β) anomeric forms based on the orientation of the hydroxyl group on the anomeric carbon.
Fructose - Structure
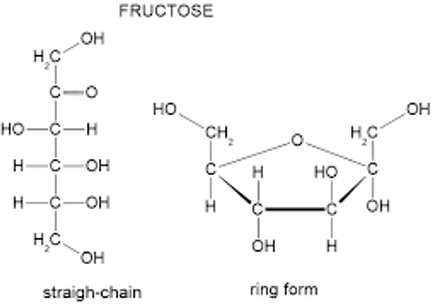
Fructose, also known as fruit sugar, has both a linear and a cyclic structure, similar to glucose. However, its structure differs as it is a ketose (contains a ketone group) rather than an aldose (contains an aldehyde group). In its cyclic form, fructose can also exhibit the α and β configurations. Glucose and Fructose both have the same molecular formula ( C 6 H 12 O 6 ), but they differ in their structural arrangement.
Amino Acids
Amino acids are organic compounds composed of an amino group (-NH ₂ ) and a carboxyl group (-COOH) attached to the same carbon atom, often referred to as the α-carbon. Beside these, the α-carbon is also bonded to a hydrogen atom and an R group (or side chain), which varies among different amino acids. There are about 20 available amino acids that makeup proteins in organisms.
They can be classified based on their side chains into polar, non-polar, acidic, and basic amino acids.
Formula:
General formula of an α-amino acid is:
H 2 N−CH(R)−COOH
where "R" represents the side chain.
Amino acids, when combined, form a bond called a peptide bond. This bond forms through a condensation reaction:
NH 2 −CHR−COOH + H 2 N−CHR ′ −COOH → NH 2 −CHR−CO−NH−CHR ′ −COOH + H 2 O
(R and R' are side chains of the amino acids)
Also Read – Cobalt II Nitrate Formula
Proteins
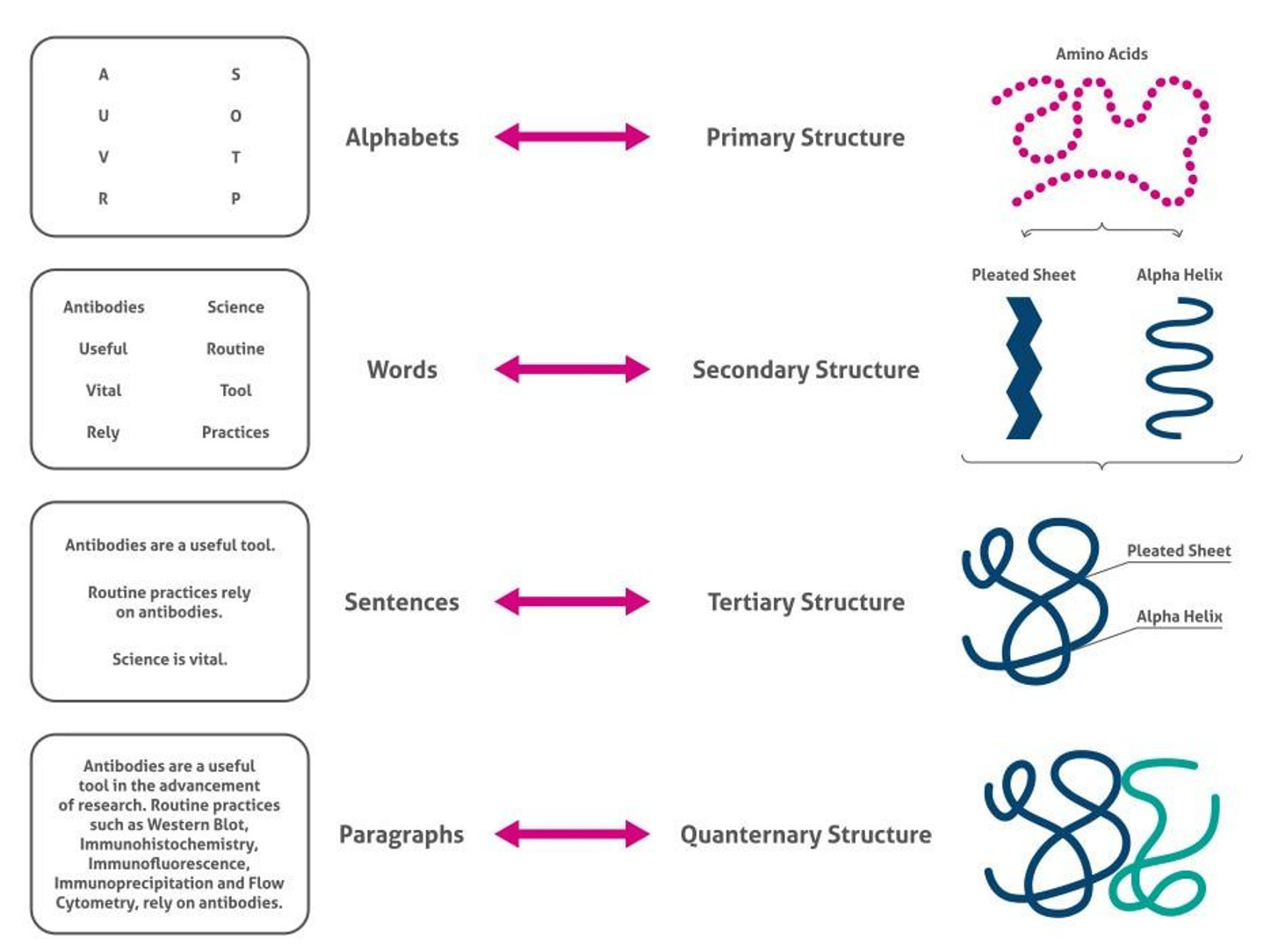
Proteins are large, complex molecules formed by the polymerization of amino acids. Their structure can be described on four levels:
Primary Structure: Linear sequence of amino acids in a protein. It's determined by the order of amino acids in the polypeptide chain.
Secondary Structure: Regular, repeating structures stabilized by hydrogen bonds. Common forms are the α-helix and the β-sheet.
Tertiary Structure: Overall 3D shape of a polypeptide chain. This structure is determined by interactions among various side chains, including hydrogen bonding, ionic interactions, and disulfide bridges.
Quaternary Structure: Structure formed by the assembly of multiple polypeptide chains. Hemoglobin, for instance, has a quaternary structure composed of 4 polypeptide chains.
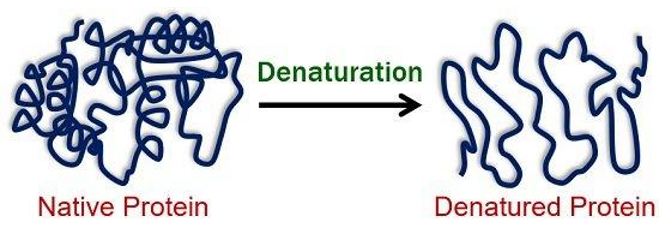
Denaturation of Proteins:
Denaturation refers to the alteration of a protein's native shape due to external stresses such as changes in pH, temperature, or the presence of chemicals. Denatured proteins lose their functional biological activity since their specific 3D structure, essential for their function, is disrupted.
For example, denaturation by heat:
Native Protein + heat → Denatured Protein for chemical denaturation
Native Protein + Chemical Denaturant → Denatured Protein
Enzyme Action:
Enzymes are biological catalysts, most of which are proteins. They speed up chemical reactions in the body without being consumed. Structure: Enzymes have a specific binding site known as the active site, where substrates bind. The enzyme's conformation facilitates the reaction for the substrate. Mechanism:
Enzymes function by reducing the activation energy required for a reaction. When a substrate binds to the active site of an enzyme, it forms an enzyme-substrate complex. The enzyme then facilitates the reaction, converting the substrate into the product.
Formula: Let E represent enzyme, S represent substrate, and P represent product. The simplified equation for enzyme action is:
Binding of Substrate to Enzyme:
E+S ⇌ ES
Formation of Product:
ES→E+P
Combining these steps,
E+S ⇌ ES→E+P
Here: E = Enzyme
S = Substrate
E−S = Enzyme-Substrate complex
P = Product
Here, E-S is the enzyme-substrate complex. This overview provides a foundation on amino acids, proteins, their denaturation, and enzyme action.
Also Check – Potassium Cyanide formula
Vitamins
Vitamins are organic molecules that are essential micronutrients. The body cannot synthesize vitamins (or not in sufficient amounts) and, therefore, they must be obtained through the diet.
Classification: Vitamins can be classified into two groups:
Fat-soluble vitamins: A, D, E, and K. They are stored in the body's fat tissues. Water-soluble vitamins: B and C.
They are not stored and need to be consumed more regularly.
Examples: Vitamin A (Retinol)
Formula: C ₂₀ H ₃₀ O
It's crucial for vision and immune system health. Vitamin C (Ascorbic Acid)
Formula: C ₆ H ₈ O ₆
It's an antioxidant and plays a role in collagen synthesis.
Also Check – Malic Acid formula
Nucleic Acids
Nucleic acids are macromolecules that store and transmit genetic information. There are two main types:
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Structure: Both are polymers of nucleotides. A nucleotide consists of a phosphate group, a five-carbon sugar (deoxyribose in DNA and ribose in RNA), and a nitrogenous base. There are four nitrogenous bases in DNA:
Adenine (A), Thymine (T), Cytosine (C), and Guanine (G). In RNA, Thymine is replaced by Uracil (U).
Formulas of Bases: Adenine: C ₅ H ₅ N ₅
Thymine: C ₅ H ₆ N ₂ O ₂
Cytosine: C ₄ H ₅ N ₃ O
Guanine: C ₅ H ₅ N ₅ O
Uracil (in RNA): C ₄ H ₄ N ₂ O ₂
DNA Structure: Double helix, where the two strands are held together by hydrogen bonds between complementary bases (A with T, C with G).
Biomolecules Formula FAQs
Q4. Which nucleic acid is single-stranded?
Q5. What is the role of hemoglobin?












