
The Cobalt II Nitrate Formula is a crucial component of Chemistry. Cobalt nitrate is an inorganic substance with the formula CO(NO3)2. This salt is made of cobalt(II). The hexahydrate Co(N O 3 ) 2 .6 H 2 O , a reddish-brown deliquescent salt soluble in water and other polar solvents, is the most prevalent form of the compound. The three-dimensional polymeric network structure of anhydrous cobalt(II) nitrate is composed of cobalt atoms that are octahedrally linked to six oxygen atoms.
Introduction To Cobalt
Before learning about the Cobalt II Nitrate Formula, one must first get familiar with cobalt. Cobalt has the chemical symbol Co and an atomic number of 27. It is a ferromagnetic, stiff, brittle, silver-white element. It is essential to create the batteries for electric cars, computers, and mobile phones. It is produced as a byproduct of processing copper and nickel ores. The letter N is used to represent nitrogen chemically. It has an atomic number of 7, 1s2 2s2 2p4 electrical configuration. Daniel Rutherford discovered nitrogen. Plants used in agriculture are significantly impacted. It makes fertilizers, nylon, nitric acid, explosives, and other products. Nitrogen is present in both soils and plant cells.Introduction To Cobalt II Nitrate
The Cobalt II An inorganic substance is nitrate. It is a cobalt salt in which nitrate serves as the counter anion, and the cobalt metal is in the +2 oxidation state. It is a colorless, odorless, solid substance that sinks and combines with water. It is employed in the creation of dyes and inks. The element symbols must first be written to formulate the cobalt II nitrate formula. The chemical element with the symbol CO is cobalt. Cobalt has a +2 charge in this chemical. Additionally, nitrate contains a negative charge. We must balance the charges to make the net charge zero and use the Criss-Cross approach. Cobalt (II) Nitrate's chemical formula is Co(N O 3 ) 2 .Also Check - Tungstic Acid formula
Cobalt II Nitrate Structure
Cobalt (II) Nitrate has the chemical formula Co(N O 3 ) 2 . Nitrogen and oxygen are nonmetals in this, but cobalt is a metal. Ionic compounds are assemblages of metals and nonmetals. Transfer of value electrons to non-cobalt (II) indicates that co has a +2 charge. Nitrate has a N O 3 - negative charge . Due to the negative charge of nitrate ions, cobalt lost electrons to them. Normally, cobalt (II) nitrate is a crystal. The following is the Lewis structure of cobalt (II) nitrate. [caption id="attachment_15789" align="alignnone" width="300"]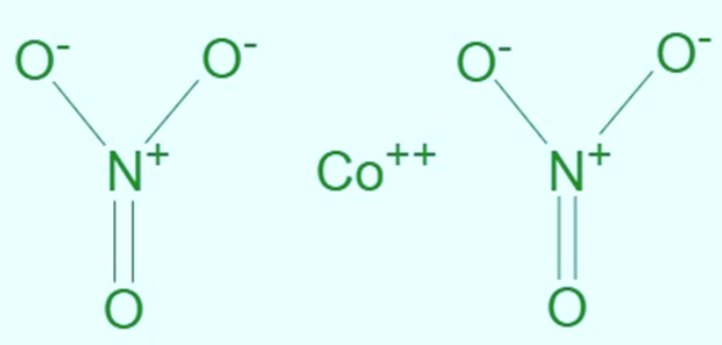 Cobalt ii nitrate structure[/caption]
Cobalt ii nitrate structure[/caption]
Cobalt II Nitrate Formula
The IUPAC designation for cobalt II nitrate formula is Co(N O 3 ) 2 . The hexahydrate form of it, Co(N O 3 ) 2 .6 H 2 O , is the most prevalent kind. It can be a reddish-brown deliquescent salt that dissolves in polar solvents like water. Cobalt II nitrate develops a three-dimensional polymeric network structure in its anhydrous state. In which six oxygen atoms, each from a unique nitrate ion, are roughly octahedrally coordinated with each cobalt(II) atom.Also Check - Charles Law Formula
Cobalt II Nitrate Formula Uses
- The manufacture of colors and inks uses nitrate.
- It is utilized to make cobalt complexes.
- It is employed to lessen the very pure cobalt.
Download PDF Colbat II Nitrate Formula
Cobalt II Nitrate Production
Treatment of metallic cobalt or one of its oxides is required to produce Cobalt Nitrate II (hexahydrate). Along with aqua fortis, it also contains hydroxides or carbonates. The reactions that follow produce cobalt nitrate II. A pressure-resistant and nitric acid corrosion-resistant container may also store metal cobalt and add industrial pure oxygen to carry out the cobalt metal dissolving reaction with the reaction temperature ranging from 20° C to 100° C . The aqua fortis concentration for the reaction was between 1mol/L and 14mol/L , and the reaction pressure was between 0.05MPa and 0.5MPa . The amount of cobalt needed for the reaction is more than the chemical equation predicts. Thus, the response might last anywhere between one and ten hours.Cobalt II Nitrate Formula FAQs
What is the purpose of cobalt II nitrate?
The creation of dyes and inks depends on it. The creation of coordination complexes such cobaloximes, carbonatotetraamminecobalt(III), and others frequently begins with cobalt(II) nitrate.
What color is the solution of cobalt II nitrate?
Reddish-brown deliquescent cobalt(II) nitrate is soluble in water and other polar solvents. Nitric acid combines with metallic cobalt or one of its oxides, hydroxides, or carbonates to generate Co(NO)3. We frequently favor using it in dyes and inks.
What type of substance is cobalt II nitrate?
Cobalt Nitrate is a crystalline form of cobalt that is extremely water soluble and suitable for nitrate- and acid-compatible applications. Each metallic nitrate is an inorganic salt of the nitrate anion and a specific metal cation.
Is cobalt II nitrate an acid or a base?
Cobalt Nitrate is a crystalline form of cobalt that is extremely water soluble and suitable for applications involving nitrates and more acidic pH levels. All metallic nitrates are inorganic salts of a specific metal cation and the nitrate anion.
Cobalt nitrate: Is it poisonous?
Skin sensitization might be brought on by prolonged or repeated contact. Asthma may be brought on by continuous or extended inhalation. The thyroid, heart, and bone marrow may all be affected by ingestion. The use of this chemical may cause human cancer.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









