CBSE Class 9 Science Notes Chapter 1: In CBSE Class 9 Science Chapter 1, "Matter in Our Surroundings," you'll learn about stuff around us. This chapter talks about different forms of matter like solids, liquids, and gases.
It explains what makes each of them unique. You'll also find out about cool things like how ice melts into water when it gets warm, or how water turns into steam when it's heated.CBSE Class 9 Science Notes Chapter 1 Matter in Our Surroundings Overview
The CBSE Class 9 Science Notes for Chapter 1 Matter in Our Surroundings provided by Physics Wallah are created by subject experts to simplify complex concepts for students. These notes provide a detailed overview of the chapter, covering fundamental topics such as the states of matter, properties of solids, liquids, and gases, and changes of state. With clear explanations and illustrative examples, students can easily grasp the concepts and enhance their understanding of the material. These notes are a valuable study aids, helping students build a strong foundation in science and excel in their academic pursuits.CBSE Class 9 Science Notes Chapter 1 PDF
The PDF link provided below for CBSE Class 9 Science Notes Chapter 1: Matter in Our Surroundings provide students a convenient resource for studying this important topic. This comprehensive notes covers important concepts such as the states of matter, physical properties, and changes of state in a clear and concise manner.CBSE Class 9 Science Notes Chapter 1 Matter in Our Surroundings
Introduction
Our surroundings are filled with various objects, from simple ones like a pencil or a table to more complex things like the food we eat or the clothes we wear. All of these are made of something called "matter." But what exactly is matter? Matter is anything that takes up space, has mass, and can be detected by our senses. In simpler terms, it's everything we can see, touch, or feel around us. The concept of matter encompasses all the substances and materials that exist in the universe.Composition of Matter
Ancient Indian philosophers delved into the nature of matter, proposing that it is composed of five fundamental constituents known as "tattvas." These tattvas, as described in our sacred books and scriptures, form the building blocks of all matter. While modern science may describe matter differently, the concept of fundamental constituents remains significant in understanding the composition of matter.Matter is made up of Particles
Matter is composed of particles. When we ponder what matter consists of, we realize that all matter, regardless of its form, is composed of extremely tiny particles. By breaking down any type of matter into smaller and smaller parts, we find that they can all be reduced to these minuscule particles. This realization leads us to the conclusion that all matter, whether it's a solid, liquid, or gas, is ultimately made up of these tiny particles. Atomic View of the Three States of Matter The atomic view of the three states of matter provides insight into how particles behave differently in solids, liquids, and gases.- Solids: In solids, particles are tightly packed together in a fixed arrangement. They vibrate around fixed positions but do not move from their places. This arrangement gives solids their definite shape and volume. The strong forces of attraction between particles hold them close together, resulting in a stable structure.
- Liquids: In liquids, particles are still close together but are not fixed in position like in solids. They have more freedom to move around each other, allowing liquids to flow and take the shape of their container. While particles in liquids have less orderly arrangement compared to solids, they still experience a moderate level of attraction to each other.
- Gases: In gases, particles are much farther apart compared to solids and liquids. They move freely and rapidly in all directions, colliding with each other and the walls of their container. Gases have neither definite shape nor volume, as they expand to fill the entire space available to them. The distance between gas particles is large, and the forces of attraction between them are weak.
Solid


Properties of Matter
The properties of matter are governed by certain characteristics shared by all its particles, as explained by the Kinetic Theory of Matter.- Composition: All matter, regardless of its form, is composed of tiny particles. These particles are the building blocks of everything around us.
- Space Between Particles: Despite being small, there is space between these particles. This space allows for movement and interaction between particles.
- Constant Motion: The particles in matter are in constant motion. They vibrate, rotate, and move around randomly within the substance, even in solids where particles appear to be stationary.
- Attraction Between Particles: There exists a force of attraction between particles, which varies depending on the state of matter. In solids, this attraction is strong, keeping particles closely packed. In liquids, it's moderate, allowing particles to move past each other. In gases, the attraction is weak, resulting in particles moving freely and independently.
Particles of Matter have space between them
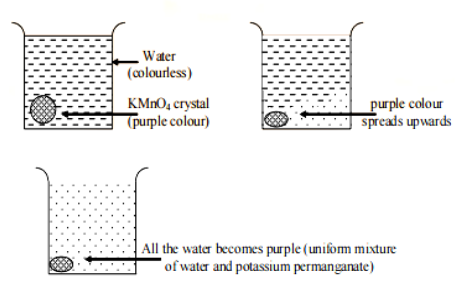
For example , if you place a crystal of potassium permanganate (which is purple in color) into a beaker filled with water, you'll observe that the purple color begins to spread throughout the water. This happens because the particles of potassium permanganate are in constant motion and move randomly in all directions.
Over time, the purple color will continue to spread until the entire solution turns purple, indicating that diffusion has occurred. This process demonstrates how substances can mix and spread out to create a uniform concentration throughout a solution.Diffusion in Gases:
Gases exhibit a very rapid rate of diffusion because their particles move swiftly in all directions. This rapid movement allows gases to spread out quickly and evenly throughout a space. For instance, when food is being cooked in the kitchen, the smell reaches us from a distance due to the fast diffusion of hot gases. On the other hand, cold food releases gases at a slower rate, so we need to be closer to it to smell it. When someone opens a bottle of perfume in a room, the scent quickly spreads throughout the space. This is because the liquid perfume evaporates into gas form, and the gas particles rapidly diffuse in all directions, mixing with the air particles and spreading across the room. Another example is the detection of a gas leak in houses using the smell of a strong chemical (ethyl mercaptan) present in cooking gas (LPG).Diffusion in Liquids:
In liquids, diffusion occurs at a slower rate compared to gases. This is because the particles in liquids move more slowly than those in gases. For instance, when a crystal of potassium permanganate is placed in water, its purple color gradually spreads throughout the water. Similarly, when ink is dropped into water, its color diffuses uniformly throughout the liquid.Solids in Liquids and Solids:
Solid-state diffusion is an extremely slow process compared to diffusion in gases and liquids. For example, when chalk particles are left on a blackboard for a long time, they become embedded in the surface, making it difficult to clean. Similarly, when two metal blocks are in contact for an extended period, particles from one metal can permeate into the other metal, illustrating solid-state diffusion.States of Matter
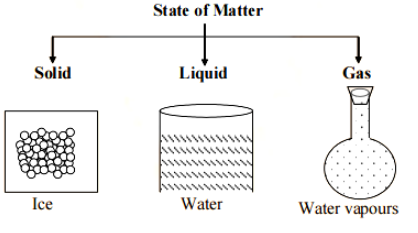
Solids: Solids have a definite volume and shape. They maintain their shape and size regardless of the container they are placed in. The particles in solids are closely packed together and have strong forces of attraction between them, making them difficult to break or compress. Examples of solids include wood, metal, and ice.
Liquids: Liquids have a definite volume but not a specific shape. They take the shape of the container they are placed in while maintaining a constant volume. The particles in liquids are loosely packed and can move around each other, allowing liquids to flow. They have weaker forces of attraction compared to solids, allowing them to be poured and shaped. Examples of liquids include water, oil, and milk.
Gases: Gases do not have a defined shape or volume. They take up all the available space in a container and expand to fill it completely. The particles in gases are far apart and move freely, colliding with each other and the walls of the container. Gases have weak forces of attraction between particles, allowing them to be easily compressed and expanded. Examples of gases include oxygen, nitrogen, and carbon dioxide.
Plasma: Plasma is a state of matter that exists at extremely high temperatures, such as in the core of the sun or stars. In this state, atoms are stripped of their electrons, resulting in a mixture of positively charged ions and a sea of free electrons. Plasma is electrically conductive and responds strongly to electromagnetic fields. It is estimated that around 99% of the observable universe is composed of plasma. Examples of plasma include lightning, flames, and the aurora borealis.
| Property | Solid | Liquid | Gas |
| Shape and volume | Fixed shape and volume | No fixed shape but has volume | Neither definite shape nor volume |
| Energy | Lowest | Medium | Highest |
| Compressibility | Difficult | Nearly difficult | Easy |
| Arrangement of molecules | Regular and closely arranged | Random and little sparsely arranged | Random and more sparsely arranged |
| Fluidity | Cannot flow | Flows from higher to lower level | Flows in all directions |
| Movement | Negligible | Depends on interparticle attraction | Free, constant and random |
| Interparticle space | Very less | More | Large |
| Interparticle attraction | Maximum | Medium | Minimum |
| Density | Maximum | Medium | Minimum |
| Rate of diffusion | Negligible | It depends on interparticle attraction. | Maximum |
Benefits of CBSE Class 9 Science Notes Chapter 1 Matter in Our Surroundings
- Clarity and Understanding: The notes provide a clear explanation of important concepts related to matter, helping students grasp the fundamentals with ease.
- Structured Learning: The notes follow a structured format, organized according to the topics covered in the chapter, which aids in systematic learning and understanding.
- Quick Reference: Students can use the notes as a quick reference guide while studying or preparing for exams, saving time and effort.
- Supplementary Resource: The notes complement the information provided in textbooks, offering additional explanations, examples, and insights to enhance understanding.
- Effective Revision: By using the notes for revision, students can reinforce their learning and ensure better retention of important concepts, leading to improved performance in exams.
CBSE Class 9 Science Notes Chapter 1 FAQs
What is matter?
What are the three states of matter?
What is diffusion?
What is the Kinetic Theory of Matter?
How does pressure affect the states of matter?