
CBSE Class 9 Science Notes Chapter 2: CBSE Class 9 Science Notes for Chapter 2, "Is Matter Around Us Pure," help students understand the basics of matter and its purity. In this chapter, students learn about different types of matter like elements, compounds, and mixtures.
They also learn how to separate mixtures using methods like filtration and distillation. The notes explain concepts like homogeneous and heterogeneous mixtures in simple terms. Students also learn about physical and chemical changes in matter and why purity is important in everyday life and industries. With easy-to-understand explanations and examples, these notes make learning about matter and its purity fun and interesting for students.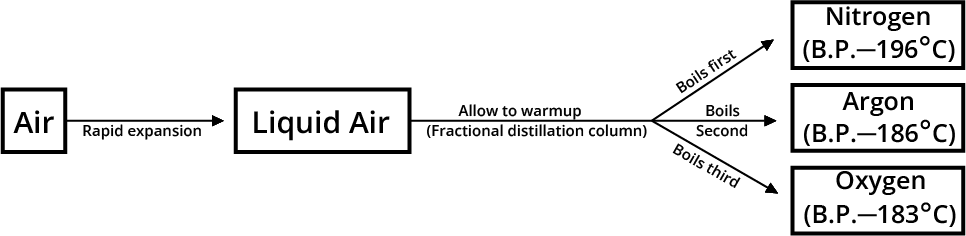
Benefits of CBSE Class 9 Science Notes Chapter 2 Is Matter Around Us Pure
- Comprehensive Coverage: These notes provide a thorough understanding of the concepts covered in the chapter, ensuring that students have a strong foundation in the subject matter.
- Clarity of Concepts: The notes present the information in a clear and concise manner, making it easier for students to grasp complex concepts and principles.
- Easy Revision: With organized and structured content, these notes facilitate quick and effective revision before exams, helping students consolidate their learning.
- Exam Preparation: The notes cover important topics, definitions, and key points that are likely to be tested in exams, thereby aiding students in their exam preparation.
- Supplementary Learning: Students can use these notes as a supplementary resource alongside their textbooks and classroom lectures, enhancing their understanding of the subject.
- Self-Assessment: The notes may include practice questions, examples, and solutions, allowing students to test their understanding and assess their progress.
CBSE Class 9 Science Notes Chapter 2 FAQs
What is Matter?
Matter is anything that occupies space and has mass. It includes solids, liquids, and gases.
What is a Pure Substance?
A pure substance is a type of matter that has a fixed composition and cannot be separated into simpler substances by physical means. Examples include elements and compounds.
What are Mixtures?
Mixtures are combinations of two or more substances that are physically combined and can be separated by physical methods. Examples include solutions, suspensions, and colloids.
What is the Difference Between a Compound and a Mixture?
A compound is a pure substance composed of two or more elements chemically combined in fixed proportions, whereas a mixture is a physical combination of two or more substances that are not chemically bonded.
What is a Colloid?
A colloid is a type of mixture in which one substance is dispersed evenly throughout another substance, resulting in a stable suspension. Colloids exhibit the Tyndall effect, where they scatter light when a beam of light is passed through them.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









