
Resonance
Chemical Bonding of Class 11
RESONANCE
There may be many molecules and ions for which it is not possible to draw a single Lewis structure. For example we can write two electronic structures of O3.
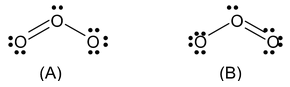
In (A) the oxygen − oxygen bond on the left is a double bond and the oxygen−oxygen bond on the right is a single bond. In B the situation is just opposite. Experiment shows however, that the two bonds are identical. Therefore neither structure A nor B can be correct.
One of the bonding pairs in ozone is spread over the region of all the three atom rather than associated with particular oxygen−oxygen bond. This delocalised bonding a type of bonding in which bonding pair of electrons is spread over a number of atoms rather than localised between two.
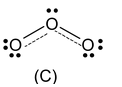
Structures (A) and (B) are called resonating or canonical structures and C is the resonance hybrid. This phenomenon is called resonance a situation in which more than one plausible structure can be written for a species and in which the true structure cannot be written at all.
Some other examples
- CO 3 2 – ion
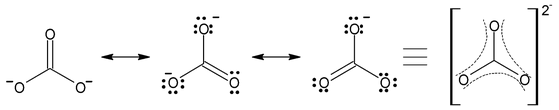
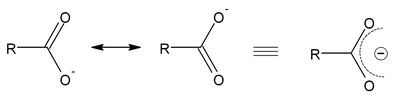
- Carbon−oxygen bond lengths in carboxylate ion are equal due to resonance.
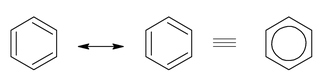
- Benzene
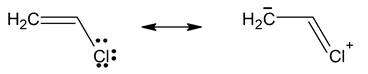
- Vinyl Chloride
Difference in the energies of the canonical forms and resonance hybrid is called resonance stabilization energy and provides stability to species.









