
Balancing Of Redox Reaction
Redox Reaction of Class 11
Some examples of the redox reactions are
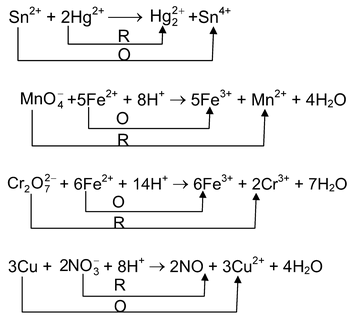
If one of the half-reactions does not take place, another half will also not take place. We can say oxidation and reduction go side by side.
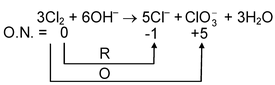
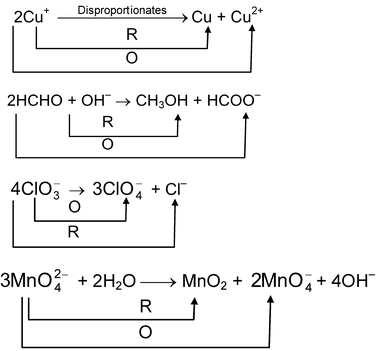
In this, we find that Cl 2 has been oxidized as well as reduced. Such type of redox reaction is called Disproportionation reaction . Examples are
We know that during redox reactions there is change in O.N. of the elements due to transference of electrons. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. It is the basic principle of balancing redox equations.
The two methods which are frequently employed are the O.N. method and the ion-electron method.
Oxidation number method
Step 1 Write the skeletal equation of all the reactants and products of the reaction.
Step 2 Indicate the oxidation number of each element above its symbol and identify the elements which change the oxidation number (O.N.).
Step 3 Calculate the increase or decrease in O.N. and identify the oxidizing and reducing agents. If more than one atom of the same element is involved. Find out the total increase or decrease in O.N. by multiplying this increase or decrease in O.N per atom by the number of atoms undergoing that change.
Step 4 Multiplying the formula of the oxidizing and the reducing agents by suitable integers to equalize the total increase or decrease in O.N. as calculated in step 3.
Step 5 Balance all atoms other than H and O.
Step 6 Finally balance H and O atoms by adding H 2 O molecules using hit and trial method.
Step 7 In case of ionic reactions
(a) For acidic medium – First balance O atoms by adding H 2 O molecules on O deficient side and then balance H atom by adding H + ions to whatever side deficient in H atoms.
(b) For basic medium – First balance O atom by adding H 2 O molecules on O excess side and then twice OH - ions on opposite side.
In all such cases evaluation of 0. N should be made by taking the contribution of covalent bonds and coordinate bonds
- When two atoms are attached with the help of a single covalent bond then its contribution for the less electronegative atom is +1 and for the more electronegative atom is –1.
- When a covalent bond is present between the two same atoms then its contribution will be zero for both the atoms
- In the case of the Coordinate bond, it gives a +2 value of oxidation number to a less electronegative atom and -2 values to a more electronegative atom when the coordinate bond is directed from a less electronegative atom to a more electronegative atom.
- If a coordinate bond is directed from a more electronegative to a less electronegative atom then its contribution will be zero for both atoms.









