Redox Reaction
Redox Reaction of Class 11
Redox Reaction
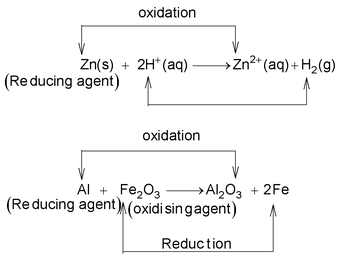
Redox reactions may be regarded as electron transfer reactions in which the electrons are transferred from one reactant to the other. The substance that loses electron is called a reducing agent or a reductant while the other which accepts the electron is called on oxidizing agent or oxidant
REDOX REACTIONS IN AQUEOUS SOLUTION
In aqueous solutions, the spontaneous redox reactions can be carried out directly as well as indirectly.
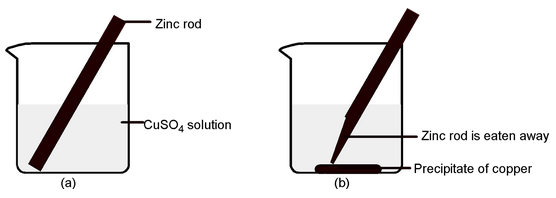
Direct Redox Reactions : Redox reactions in which oxidation and reduction takes place in the same vessel are called direct redox reactions. In such reactions, the transfer of electrons from reductant to oxidant occurs over a very short distance (within molecular diameters). For example, if a zinc rod is placed in a solution of copper sulphate in a beaker, a spontaneous reaction occurs and following changes will be observed.
Observations for redox reaction occurring in a beaker
- Zinc rod starts dissolving and loses its mass gradually.
- The blue color of the solution starts fading.
- Copper metal either starts settling at the bottom of the beaker or depositing on the zinc rod.
- The reaction is exothermic i.e., it takes place with the evolution of heat.
- The solution remains electrically neutral throughout.
- The reaction will not continue indefinitely but stops after some time.
The overall reaction taking place in the beaker may be represented as:
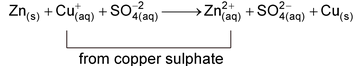
Discarding the common SO 4 2– ions
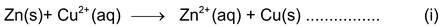
Zinc loses electrons and changes to Zn 2+ ions which go into the solution. As a result, the mass of zinc rod decreases. The electrons lost by zinc rod are gained by Cu 2+ (aq) ions and they change into Cu(s) atoms which settle down at the bottom of beaker in the form of precipitate. It may be noted that in the direct redox reaction, the chemical energy appears as heat.
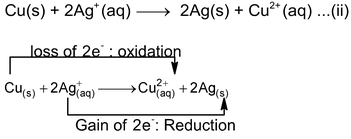
Similarly, when we dip a copper strip in a silver nitrate solution, copper gets oxidised and go into the solution whereas Ag
+
ions accept electrons and get reduced. The reaction may be written as:
|
|
Thus, copper is oxidised to Cu 2+ and Ag + is reduced to Ag(s). Therefore, copper acts as reducing agent or reductant while silver acts as oxidising agent or oxidant in equation (ii) and copper acts as an oxidising agent in equation (i). The reason being that electron−donating ability of zinc is more than that of Cu while electron donating ability of Cu is more than that of Ag.
Oxidation and reduction half reactions
Every redox reaction can be split up into two half – reactions one representing loss of electron i.e. oxidation half reaction while the other representing gain of electrons i.e. reduction half reaction like

Further Reading :