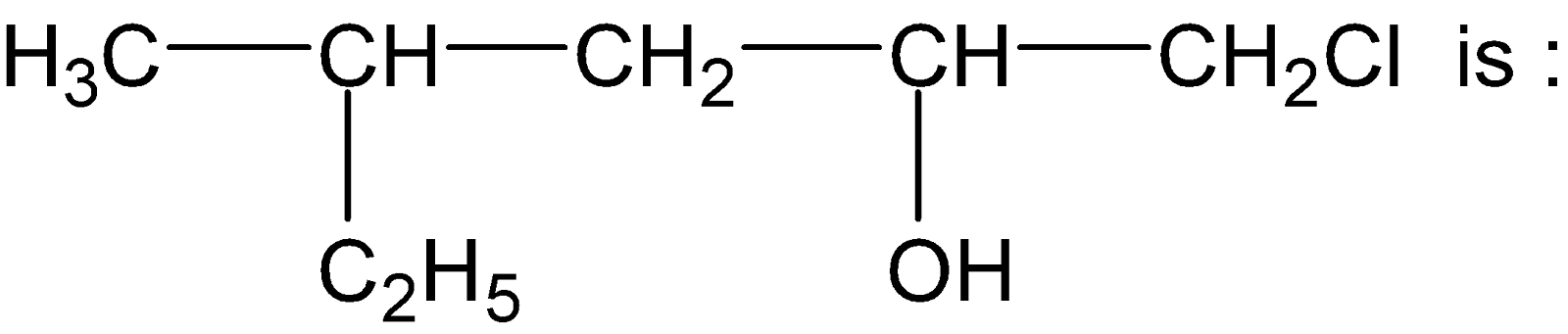
Frequently Asked Questions
Li shows diagonal relationship with
A: Mg
B: Be
C: Al
D: B
Solution:
Explanation:
Diagonal relationship between elements refers to the relationship between diagonally adjacent elements in the second and third periods due to similarity in their size.
Lithium is different from other alkali metals due to its small size but since its size resembles with that of magnesium, it shows a diagonal relationship with magnesium.
Final Answer:
The correct option is A: Mg
How many moles of magnesium phosphate
Mg 3 (PO 4 ) 2 will contain 0.25 mole of oxygen atoms ?
.png----FileNotFound----)
Solution:
Explanation:
.png----FileNotFound----)
Final Answer:
The correct option is B: 3.125×10 -2 .
Draw the molecular structure of
N 2 O 5 .
Solution:
Explanation:
.png----FileNotFound----)
Aqueous solution of borax is
A: neutral
B: amphoteric
C: basic
D: acidic
Solution:
Explanation:
Borax (Na[ B 4 O 5 (OH) 4 ].8H 2 O) is a salt of boric acid (H 3 BO 3 ) and sodium hydroxide (NaHO) base.
Borax, also called sodium borate, sodium tetraborate or disodium tetraborate is basically a salt of weak acid and a strong base.
Borax, when dissolved in water, ionizes and hydrolysis of borate ion formed gives HO - and boric acid.
Since boric acid is weak acid and sodium hydroxide is strong, the aqueous solution is alkaline in nature.
Final Answer:
The correct answer is C: basic
The most abundant element in the universe is
A: helium
B: oxygen
C: silicon
D: hydrogen
Solution:
Explanation:-
- Hydrogen is the most abundant element in the universe; it is present in every Star.
- Two hydrogen atoms fuse together to form Helium atom and this Helium atom is the second most abundant element.
- Oxygen is the third most abundant element .
Final Answer:-
The most abundant element in the Universe is (D) hydrogen