
Chlorous Acid Formula: Chlorous acid, represented by the chemical formula HClO 2 , falls within the category of inorganic compounds. It is characterized as a relatively weak acid. In this acid, chlorine exhibits an oxidation state of +3. However, the pure form of chlorous acid is inherently unstable, tending to undergo a process known as disproportionation. This process results in the formation of hypochlorous acid, in which chlorine holds an oxidation state of +1, and chloric acid, where chlorine's oxidation state is +5. This transformation is depicted by the following chemical equation:
2 HClO 2 → HClO + HClO 3
Obtaining pure chlorous acid is a challenging task. But, its conjugate base, referred to as chlorite, is relatively stable. One prominent example of a salt containing this anion is sodium chlorite, a substance widely recognized in chemistry. These chlorite-based salts, as well as their related counterparts, are occasionally used in the production of chlorine dioxide.
Chlorous Acid Formula
Chlorous acid formula is HClO 2 .Chlorous acid is classified as an inorganic compound, exhibiting characteristics of a weak acid. Within this acid, chlorine maintains an oxidation state of +3. However, the pure form of chlorous acid is inherently unstable and tends to undergo a disproportionation reaction, resulting in the formation of hypochlorous acid and chloric acid. Due to its instability, obtaining pure chlorous acid can be quite challenging.
This acid possesses a stable conjugate base known as chlorite, which is also referred to as Chloride Secure. To synthesize this compound, one can react with barium or lead chlorite with dilute sulfuric acid. The chemical composition of chlorous acid consists of one chlorine atom, one hydrogen atom, and two oxygen atoms.
Chlorous Acid Formula Structure
Chlorous acid formula is HClO 2 . Chlorous acid has a molar weight of 68.46 grams per mole. Its structure is formed through the attachment of one proton (H+) to a chlorite anion (ClO 2 - ).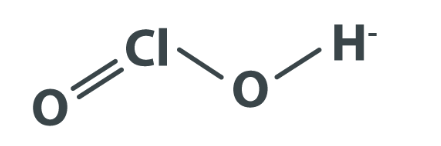
Chlorous Acid Formula Physical Properties
Below are the some physical properties of Chlorous Acid.
- Chlorous acid formula is HClO 2
- Chlorous acid is a colorless liquid.
- Chlorous acid acts as a strong oxidizing agent.
- Chlorous acid is also incompatible with reducing agents and alkalis.
- Chlorous acid is commonly used as a mouthwash for the reduction of plaque.
- It has a 1.96 pKa and its determination takes place as the stability of the conjugate base of an acid is determined. The value of 1.96 pKa indicates that the conjugated base chlorite ClO 2 - is stable.
- Chlorous acid boiling point is 502.07°C.
Chlorous Acid Formula Chemical Properties
Chlorous acid formula is HClO 2 . Chlorous acid is unstable and when it reacts gives product chloric acid and hypochlorous acid. one production due to the reduction of Cl 3 + to Cl 1 + , while the second production due to the oxidation of Cl 3 + to Cl 5 + 2HClO 2 → HClO + HClO 3 Chlorous acid acts as a strong oxidizing agent. Chlorous acid has a tendency to undergo disproportionation due to its oxidative potential. Chlorine is the only halogen that gives the production of an isolable acid of formula HXO 2 .Uses of Chlorous Acid
Below are some uses of Chlorous acid.
Chlorous acid acts as a strong oxidizing agent.
Chlorous acid is also incompatible with reducing agents and alkalis.
Chlorous acidis commonly used as a mouthwash for the reduction of plaque.
This particular salt finds applications in various industrial processes, such as the production of chlorine dioxide.
Chlorous acid is an inorganic compound characterized by its weak acidity and instability in its pure form. which leads to disproportionation, and production of hypochlorous acid and chloric acid. It holds a stable conjugate base known as chlorite. Chlorous acid is a colorless liquid with potent oxidizing properties and is used in various applications as an oxidizing agent, used as mouthwash for the reduction of plaque.
Join Online Course of Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your chemistry knowledge. and build a strong foundation.
| Related Links | |
| Aluminium Phosphate Formula | Ammonia Formula |
| Cobalt (II) Sulfate Formula | Tin (IV) Chloride Formula |
Chlorous Acid Formula FAQs
What is the chemical formula for chlorous acid?
What is the oxidation state of chlorine in chlorous acid?
What is disproportionation in the context of chlorous acid?
Why is obtaining pure chlorous acid challenging?
What is the stable conjugate base of chlorous acid called?










