
Tin IV Chloride Formula: Tin (IV) chloride is composed of two distinct elements: Tin and Chlorine. Tin, a lustrous silver-colored and malleable metal. It is situated within group 14 on the periodic table. It serves various purposes, including plating steel cans and acting as a material for food containers.
With an atomic number of 50 and denoted by the symbol Sn, it's an essential element in various applications. On the other hand, Chlorine is a corrosive and toxic gas found within group 17 on the periodic table, being the second lightest element in that group. It possesses an atomic number of 17 and is represented by the symbol Cl.
Tin IV Chloride Formula
Tin (IV) chloride formula is SnCl 4 . It is also known as Stannic Chloride, is an inorganic compound that appears as a colorless to light yellow fuming liquid. It was initially identified by Andreas Libavius. Notably, this substance is hygroscopic, meaning it readily absorbs moisture from the air and emits fumes as a result. Its IUPAC names include Tetrachlorostannane, Tin (IV) chloride, and Tin tetrachloride.
Tin IV Chloride Formula Structure
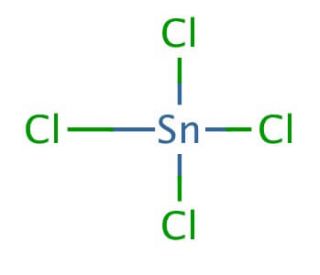
Tin IV Chloride Formula is SnCl 4 . Tin (IV) chloride has a specific structure that can be described here. At the atomic level, it consists of a central tin atom bonded to four chlorine atoms. The bond between the tin and chlorine atoms involves strong covalent bonds.
Preparation of Tin IV Chloride
Tin IV Chloride Formula is SnCl 4 . To produce Tin (IV) chloride, one can follow several methods:
Direct Reaction with Chlorine Gas: Tin (IV) chloride can be obtained by reacting elemental Tin with Chlorine gas at a temperature of 115 °C. Below are chemical reactions as follows.
Sn + 2Cl 2 →SnCl 4
Decomposition of Potassium Hexachlorostannate: By subjecting Potassium hexachlorostannate to high heat, it decomposes into Tin (IV) chloride and Potassium chloride:
K 2 SnCl 6 → SnCl 4 + 2KCl
Preparation of Hydrated Tin (IV) Chloride (Pentahydrate): The hydrated form of Tin (IV) chloride. It is known as Pentahydrate (SnCl 4 .5H 2 O), and can be produced by oxidizing Tin(II) chloride (SnCl 2 ) in a solution using Potassium nitrate (KNO 3 ). This reaction results in the formation of the hydrated compound.
These methods offer various routes to obtaining Tin (IV) chloride for different applications and chemical requirements.
Tin IV Chloride Formula Physical Properties
Physical Properties of Tin (IV) chloride
Tin IV Chloride Formula is SnCl 4 .
It appears in the form of colorless/light yellow fuming liquid.
Its molecular weight is 260.5 g/mol.
Tin (IV) chloride melting point is 33 °C in anhydrous form and 56 °C in pentahydrate form.
Its boiling point is 114 °C.
Tin (IV) chloride Density of anhydrous Tin (IV) chloride is 2.226 g/cm3 and for pentahydrate, it is 2.04 g/cm3.
Tin (IV) chloride is soluble in water.
Tin IV Chloride Formula Chemical Properties
Tin IV Chloride Formula is SnCl4. The chemical properties of Tin (IV) chloride can be summarized as follows:
Reaction with Grignard Reagents: Tin (IV) chloride can react with Grignard reagents (RMgCl) to produce tetra alkyl tin compounds.
SnCl 4 + 4RMgCl →Sn R4 + 4MgCl 2
Redistribution Reactions: In redistribution reactions, Tin (IV) chloride is used in reactions with tetra organotin compounds, leading to the formation of two molecules of dichloro organotin compounds.
SnCl 4 + SnR 4 → 2SnCl 2 R 2
Fuming in Humid Air: Anhydrous Tin (IV) chloride (SnCl 4 ) emits fumes when exposed to humid air, resulting in the formation of a white smoke consisting of Tin (IV) oxide (SnO 2 ) and Hydrogen chloride (HCl). The chemical reaction for this process is:
SnCl 4 + 2H 2 O→SnO 2 + 4HCl
These chemical reactions illustrate the reactivity and behavior of Tin (IV) chloride in various contexts and its ability to form different tin compounds under specific conditions.
Uses of Tin IV Chloride
Tin (IV) chloride finds various applications, including:
Tin (IV) chloride has a diverse range of applications, serving as a catalyst and reagent in chemical synthesis, aiding in tin electroplating, contributing to textile dyeing and printing, playing a role in specialty glass manufacturing, and occasionally appearing in medicinal and pharmaceutical applications. Additionally, it is involved in pigment production, metallurgical processes as a reducing agent, and the synthesis of various tin compounds.
| Related Links | |
| Strontium Chloride Formula | Barium Bromide Formula |
| Barium Fluoride Formula | Hydrobromic Acid Formula |
Tin IV Chloride Formula FAQs
What is Tin (IV) chloride?
What are the common uses of Tin (IV) chloride?
Is Tin (IV) chloride safe to handle?
Can Tin (IV) chloride be used in food packaging?
How is Tin (IV) chloride prepared?










