
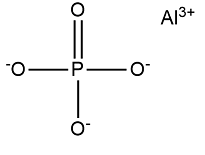
Aluminium phosphate formula is AlPO 4 . Aluminium phosphate consists of 1 Phosphorus atom, 4 Oxygen atoms, (3 shared with single bond & 1 with double bond), and 1 Aluminum atom. Mostly it is present as dihydrate means Aluminium phosphate along with 2 water ions as well as pentahydrate means Aluminum phosphate along with 5 water ions.
Aluminium phosphate is an amorphous form of aluminium hydroxy phosphate in this some of the hydroxyl groups of aluminium hydroxide are replaced by phosphate groups and it is used like flame retardant,, fungicide,Antacid, catalyst food added substance, etc.
It is generally present as an anhydrous salt and contains dihydrate and Pentahydrate structures.
Dihydrate: AlPO 4 .2H 2 O
Pentahydrate: AlPO 4 .5H 2 O
Aluminium Phosphate Formula Structure
Aluminium phosphate formula is AlPO 4 . Aluminium phosphate dihydrate has a group of phosphate anions, aluminium cations, and water. It contains 1 Phosphorus atom, 4 Oxygen atoms. Generally it appears as dihydrate means Aluminium phosphate along with 2 water ions as well as pentahydrate means Aluminum phosphate along with 5 water ions.
Aluminium Phosphate Formula Physical Properties
Aluminium phosphate formula is AlPO 4Aluminium phosphate is a white crystalline powder.
Aluminium phosphate is a colorless liquid in aqueous form.
Aluminium phosphate is a foul odour.
Aluminium phosphate density is 2.56 g.cm3 in solid-state.
Aluminium phosphate molar mass is 121.9529 g/mol.
Aluminium phosphate refractive index is 1.546.
The Melting point of Aluminum phosphate is 1800°C.
Aluminium phosphate is decomposed at its Boiling point.
Aluminium phosphate is Insoluble in water.
Aluminium phosphate is very slightly soluble in HCl & HNO 3 .
Aluminium Phosphate Formula Chemical Properties
Aluminium phosphate formula is AlPO 4 . Aluminium phosphate reacts with Hydrochloric acid (HCL). Then this reaction produces Aluminium trichloride (AlPO 4 ) and Phosphoric acid (H 3 PO 4 ). Below is the chemical reaction.
AlPO 4 + 3HCl → AlCl 3 + H 3 PO 4
Aluminium phosphate reacts with Magnesium chloride (MgCl 2 ). This reaction produces Aluminium trichloride (AlCl 3 ) and Magnesium phosphate (Mg 3 (PO 4 ) 2 . Below is the chemical reaction.
2AlPO 4 + 3MgCl 2 → Mg 3 (PO 4 ) 2 + 2AlCl 3
Aluminium Phosphate Uses
Molecular sieves, commonly referred to as "ALPOs," encompass various types of aluminum phosphate molecular sieves. These materials were initially documented in 1982 and share a common chemical composition of AlPO 4 . They exhibit framework structures with micro-porous cavities, composed of alternating AlO 4 and PO4 tetrahedra. Notably, the crystalline berlinite, which lacks cavities, also features this alternating arrangement of AlO 4 and PO 4 tetrahedra.
The distinguishing factor among these aluminophosphate frameworks lies in the relative orientation of the AlO 4 and PO 4 tetrahedra, resulting in the formation of cavities of different sizes. In this aspect, they bear similarity to aluminosilicate zeolites, although the latter possess electrically charged frameworks. The typical preparation of an aluminophosphate involves a hydrothermal reaction between phosphoric acid, aluminum in the form of hydroxide, and an aluminum salt, such as aluminum nitrate salt or alkoxide. This reaction occurs under controlled pH conditions and in the presence of organic amines, which serve as templates or structure directing agents (SDAs) guiding the formation of the porous framework.
In another context, aluminum phosphate, along with aluminum hydroxide, serves as a common immunologic adjuvant in vaccinations. The widespread use of aluminum adjuvants is attributed to their cost-effectiveness, extensive history of safe use, and efficiency with most antigens. However, the specific mechanisms by which these salts function as adjuvants remain uncertain.
Similarly to aluminum hydroxide, AlPO4 finds application as an antacid, neutralizing stomach acid (HCl) by forming AlCl 3 . It's worth noting that up to 20% of aluminum from ingested antacid salts can be absorbed from the gastrointestinal tract. Despite some unverified concerns about potential neurological effects of aluminum, aluminum phosphate and hydroxide salts are generally regarded as safe for use as antacids, even during pregnancy and breastfeeding.
Furthermore, AlPO 4 has additional uses, either in combination with other compounds or on its own. These applications include its role as a white colorant in pigments, corrosion inhibitor, and in cements, including dental cements. Similar compounds, such as Al(H 2 PO 4 ) 3 and Al(H 2 PO 4 )(HPO 4 ), are also employed in dental cements, metal coatings, glaze compositions, and as refractory binders and adhesives.
| Related Links | |
| Copper I Chloride Formula | Copper (II) Chloride Formula |
| Ammonium Bicarbonate Formula | Copper Sulfate Formula |
Aluminium Phosphate Formula FAQs
What is the chemical formula of Aluminium phosphate?
What is the common name of aluminium phosphate?
What are the physical properties of aluminium phosphate?
What are some common uses of Aluminium Phosphate?










