
Cobalt II Sulfate Formula: Cobalt(II) sulfate, alternatively known as cobaltous sulfate or cobalt sulfate, is classified as a hazardous metallic salt. This inorganic compound possesses the chemical formula CoSO 4 or CoO 4 S and comprises cobalt in a +2 oxidation state, combined with sulfate in a 1:1 ratio. It presents as a scentless, rose-pink solid, which readily submerges and dissolves in boiling water. Cobalt(II) sulfate exhibits different hydrate forms, including heptahydrate, hexahydrate, and monohydrate. Among these, the heptahydrate variant is the most commonly accessible form of cobalt salt. It demonstrates insolubility in ammonia and solubility in methanol.
Upon heating to 735 °C, it decomposes, releasing toxic sulfur oxide fumes. This compound finds utility in various applications, such as pigment manufacturing, catalysis, as a drying agent in paint and ink, and as a soil amendment. Additionally, it serves as a supplement for addressing Vitamin B12 deficiency and is utilized in storage batteries. It is important to note that exposure to cobalt(II) sulfate can lead to irritation in the eyes, skin, and respiratory tract, with potential effects on the lungs, heart, and kidneys.
Cobalt II Sulfate Formula Structure
Cobalt (II) Sulfate has the chemical formula CoSO 4 or CoO 4 S. Its structure consists of a cobalt ion (Co²⁺) bonded to a sulfate ion (SO₄²⁻) in a 1:1 ratio.
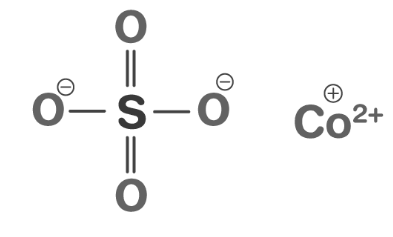
Cobalt II Sulfate Formula Physical Properties
Below are the physical properties of Cobalt II Sulfate like chemical formula, IUPAC Name, Appearance, Odour, Melting Point, Molar Mass and Density.
| Cobalt II Sulfate Formula Properties | |
| Chemical formula | CoSO 4 ·(H 2 O)n (n=0,1,6,7) |
| IUPAC Name | Cobalt(II) sulfate |
| Appearance | Reddish crystalline (anhydrous, monohydrate) pink salt (hexahydrate) |
| Odour | Odourless |
| Melting point | 735 °C |
| Molar mass | 154.996 g/mol (anhydrous) 173.01 g/mol (monohydrate) 263.08 g/mol (hexahydrate) 281.103 g/mol (heptahydrate) |
| Density | 3.71 g/cm3 (anhydrous) 3.075 g/cm3 (monohydrate) 2.019 g/cm3 (hexahydrate) 1.948 g/cm3 (heptahydrate) |
Cobalt II Sulfate Formula Chemical Properties
Cobalt II Sulfate Formula is CoSO 4 . When the temperature range rises between 600-700℃. Then Cobalt Sulfate decomposes into oxygen, sulfur dioxide and cobaltous oxide.
2CoSO 4 → O 2 + 2SO 2 + 2CoO
When Cobalt(II) Sulfate reacts with sodium hydroxide. Then it produces sodium sulfate and Cobalt(II) hydroxide.
2NaOH + CoSO 4 → Na 2 SO 4 + Co(OH) 2
When Cobalt(II) Sulfate reacts with Aluminium hydroxide. Then it produces e cobalt(II) aluminium oxide, sulfur dioxide, oxygen, and water.
4Al(OH) 3 + 2CoSO 4 → 2CoAl 2 O 4 + 2SO 2 + O 2 + 6H 2 O
When Cobalt(II) Sulfate reacts with concentrated solution of ammonium hydroxide. Then hexaamminecobalt(II) sulfate and water are produced.
6NH 4 OH + CoSO 4 → 6H 2 O + [Co(NH 3 ) 6 ]SO 4
When Cobalt(II) Sulfate reacts with lead nitrate. Then lead sulfate and cobalt(II) nitrate are produced.
CoSO 4 + Pb(NO 3 ) 2 → PbSO 4 + Co(NO 3 ) 2
Preparation of Cobalt (II) Sulfate
Cobalt II Sulfate Formula is CoSO 4 . Cobalt (II) Sulfate can be produced. When aqueous sulfuric acid reacts with cobaltous oxide.
CoO + H 2 SO 4 + 6H 2 O → CoSO 4 (H 2 O) 7
Metallic cobalt, its hydroxide, or carbonate react with aqueous sulfuric acid. Then the byproduct is Cobalt (II) Sulfate formed.
Co + H 2 SO 4 + 7H 2 O → CoSO 4 (H 2 O) 7 + H 2
At Room Temperature, if humidity is below 70% . Then heptahydrate transforms into hexahydrate. While if temperature rises between the range of 100 and 250 °C then hexahydrate transforms into the monohydrate and anhydrous forms.
CoSO 4 (H 2 O) 7 → CoSO 4 (H 2 O) 6 + H 2 O
CoSO 4 (H 2 O) 6 → CoSO 4 (H 2 O) + 5H 2 O
CoSO 4 (H 2 O) → CoSO 4 + H 2 O
Uses Cobalt (II) Sulfate
Hydrated cobalt(II) sulfate finds several valuable applications:
In the field of electroplating, it plays a vital role, helping to deposit a layer of cobalt onto various surfaces, enhancing their durability and appearance.
It contributes to the manufacturing of storage batteries, playing a part in the development of efficient energy storage solutions.
This compound acts as a catalyst in various chemical reactions, speeding up the process without being consumed in the reaction.
It is used as a drier in the production of paints and inks, facilitating their faster drying and setting.
In agriculture, it serves as a soil additive, aiding in the enrichment of soil with essential nutrients.
Interestingly, cobalt (II) sulfate is used in veterinary medicine to prevent and treat cobalt deficiency in ruminants, ensuring their overall health and well-being.
Health Hazards
While cobalt is indeed an essential mineral for mammals, excessive intake, even just a few micrograms per day, can have adverse effects.
Contact with cobalt can lead to irritation of the eyes and skin.
Inhaling cobalt can result in breathing difficulties, coughing, and potential issues with the respiratory tract.
When ingested, it may cause pain and vomiting, with the potential for harm to vital organs such as the kidneys, lungs, and heart.
| Related Links | |
| Arsenic Acid Formula | Strontium Sulfate Formula |
| Strontium Chloride Formula | Barium Bromide Formula |
Cobalt II Sulfate Formula FAQs
What is the chemical formula for Cobalt(II) Sulfate?
What does Cobalt(II) Sulfate look like?
Does Cobalt(II) Sulfate have an odour?
At what temperature does Cobalt(II) Sulfate melt?
What is the molar mass of Cobalt(II) Sulfate?










