
A deep blue compound with a chemical formula N 2 O 3 , dinitrogen trioxide is formed when nitrogen dioxide and nitric oxide are mixed and cooled below 21°C. Learn in detail about the dinitrogen trioxide formula and its chemical structure and properties in this article. It is a powerful oxidizer that is highly toxic and corrosive.
Dinitrogen Trioxide Properties
| Properties of Dinitrogen Trioxide | |
| Name | Dinitrogen trioxide |
| Appearance | Deep Blue tinted gas |
| Chemical Formula | N 2 O 3 |
| Boiling Point | 3.5 °C |
| Melting Point | −100.7 °C |
| Density | 1.4 g/cm³ (liquid) 1.783 g/cm3 (gas) |
| Molar mass | 76.01 g/mol |
| Solubility in Water | Soluble |
Dinitrogen Trioxide Chemical Structure
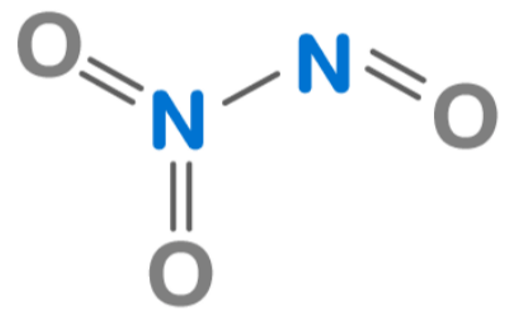
Dinitrogen trioxide is a planar molecule composed of two nitrogen atoms bonded by a single bond. One nitrogen atom is bonded to one oxygen atom. The other two oxygen atoms are bound to another nitrogen atom.
Dinitrogen Trioxide Uses
Despite its highly combustible nature, dinitrogen trioxide does not burn. It is mostly used as an oxidizing agent when combined with other chemical compounds.
Also Check – Molecular Speed Formula
Structure of Nitrogen Trioxide
The length of N–N bonds in hydrazine is about 145 pm, similar to that in nitrogen. However, dinitrogen trioxide has an unusual long N–N bond of 186 pm. Other examples of long N–N bonds can be found in nitrogen oxides such as dinitrogen tetroxide (175 pm). The Nitrogen Trioxide molecule has a planar structure with Cs symmetry and can produce unstable nitrous acid (HNO 2 ) when mixed into water. Although there is an alternative structure of O=N–O–N=O for the true anhydride, it is not observed. If the produced nitrous acid is not used quickly, it will decompose into nitric oxide and nitric acid. In some cases, adding N 2 O 3 to solutions of certain bases like NaOH can result in the production of nitrite salts.
Also Check – Partial Pressure Formula
Handling and Storage
To avoid any potential risks, it is recommended to wear fully encapsulating, vapour-protective garments in the event of non-fire spills and leaks. Interacting with the spilled material should be avoided, as well as keeping any flammable items away from the affected area. If safe to do so, try to stop the leak and use water spray to minimize vapours or redirect their drift. However, make sure not to let spilt substances come into contact with water runoff. Additionally, do not direct water towards the spill or source of the leak. If possible, flip leaky containers so that gas rather than liquid escapes. It is also important to restrict access to rivers, sewers, basements and other confined spaces until the gas has dissipated. Adequate ventilation in the area should be ensured for safety purposes.
Dinitrogen Trioxide Formula FAQs
Q1. What is dinitrogen trioxide?
Q2. How is dinitrogen trioxide formed?
Q3. What are the properties of dinitrogen trioxide?
Q4. What are the uses of dinitrogen trioxide?
Q5. Is dinitrogen trioxide dangerous?










