
Faradays Laws of Electrolysis: Faraday's electrolysis laws are established quantitative relationships derived from the electrochemical research conducted by Michael Faraday in 1833.
What is an Electrode?
An electrode can be defined as the specific location where electric current either enters or exits the electrolyte or circuit. When current leaves the electrode. It is referred to as the cathode, whereas when current enters the electrode, it is called the anode.
Electrodes constitute a fundamental element in electrochemical cells. It is imperative for an electrode to exhibit excellent electrical conductivity. Notably, inert electrodes also exist, which do not actively participate in the chemical reactions. Electrodes can be composed of various materials such as gold, platinum, carbon, graphite, and metals. They serve as surfaces for facilitating oxidation-reduction reactions within the cells.
Faradays Laws of Electrolysis
Electrodes can be primarily categorized into two types:
Reactive electrodes are actively involved in the cell's reaction and may dissolve in the electrolyte. Examples of reactive electrodes include copper, silver, zinc, and others, which are commonly employed in potentiometric experiments.
Inert electrodes, on the other hand, do not partake in the chemical reactions. Examples of inert electrodes comprise carbon and platinum electrodes.
Faradays Laws of Electrolysis First Law
MIchael Faraday’s discovery states that the mass of a substance deposited at an electrode is directly proportional to charge. SI units are ampere seconds or coulomb's. Equations are represented below.
In equation (1):
"m" represents the mass of a substance (measured in grams) deposited or released at an electrode.
"Q" signifies the quantity of electric charge (in coulombs) or electricity that has passed through it.
When we eliminate the proportionality from equation (1) –
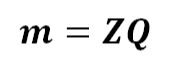
In this equation. The constant of proportionality is denoted as Z. In the term of electro chemical equivalent (ECE) of the substance. Electro chemical equivalent represents the amount of substance deposited per unit electric charge.
Faradays Law of Electrolysis Second Law
Faraday's Second Law of Electrolysis asserts that the quantity of substance deposited at an electrode when a specific amount of charge is passed through it is directly proportional to its chemical equivalent weight. In other words, if the same amount of electricity is passed through different electrolytes. The mass of the substances deposited is proportionate to their respective chemical equivalents or equivalent weights. This relationship can be shows as follows:
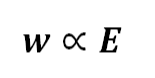
“w” denotes the mass of the substance.
“E” denotes the equivalent weight of the substance.
This relationship can also be represented as:
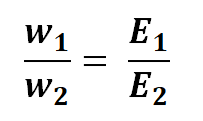
The equivalent weight, also known as the chemical equivalent, of a substance can be defined as the result of dividing its atomic weight by its valency. This relationship can be shows as:
Equivalent weight = Atomic weight / Valency
Faraday's Second Law of Electrolysis can be further clarified through the following illustration:
Three distinct chemical reactions taking place in three separate electrolytic cells connected in series. In the first electrolytic cell, sodium ions acquire electrons and transform into sodium.
Na + + e − → Na
In the 2nd electrolytic cell, the following reaction takes place:
Cu +2 + 2e − → Cu
In the 3rd electrolytic cell, the following reaction takes place:
Al 3+ + 3e − → Al
Assuming that "y" moles of electrons traverse the three cells, the quantities of sodium, aluminum, and copper released amount to 23y grams, 9y grams, and 31.75y grams, respectively.
It's important to note that one mole of electrons is necessary for the reduction of one mole of ions. As a point of reference, the charge carried by a single electron is equal to:
1.6021×10-19 C
and one mole of electrons is correspond to
6.023×1023
electrons. So, charge on one mole of electrons is correspond to
(6.023×1023)×(1.6021×10-19C)=96500 C
This charge (96500 C) is called 1 Faraday.
If 1 Faraday of charge is delivered to an electrolytic cell, it results in the deposition of 1 gram of the substance's equivalent weight.we can represent this relationship as follows:
w=(Q / 96500)×E
On Adding the 1st and 2nd laws we get –
Z= E / 96500
Application of Faradays Laws of Electrolysis
The practical uses of Faraday's Laws of electrolysis encompass:
Removal of metal ions from an aqueous solution.
Electrolysis in redox reactions.
Electrolysis in the manufacturing of heavy water.
Electrolysis in the manufacturing of galvanic cells and fuel cells.
Electrolysis is used to avoid metal corrosion by using the technique of electroplating.
Enroll Now Online Course for Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your chemistry knowledge. and build a strong foundation.| Related Links | |
| Fluorine Gas Formula | Potassium Hexacyanoferrate (III) Formula |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
Faradays Laws of Electrolysis FAQs
What are Faraday's Laws of Electrolysis?
What is an electrode in electrolysis?
What is the first law of electrolysis?
What is the second law of electrolysis?










