Hydrogen sulfate formula is HSO 4 .Hydrogen Sulfate, also known as bisulfate. It is an ion derived from sulfuric acid. The chemical formula of hydrogen sulfate signifies its composition. It consists of one hydrogen atom, one sulfur atom, and four oxygen atoms. Its ion bears a charge of '-1'.
Hydrogen plays an important role in the constitution of water, represented by its chemical symbol H and occupying the topmost position on the periodic table. It is a fundamental element in the formation of water when it combines with oxygen. Hydrogen holds an atomic mass of approximately 1.007825 g.mol-1 and an atomic number of 1. It carries a single unit of positive charge and has one electron in its nucleus.
Sulfate or Sulphate, represents an ion, being a salt derived from sulfuric acid. It has a molar mass of approximately 96.06 g/mol. Sulfate is water-soluble and is a divalent anion formed through the oxidation of elemental sulfur and organic sulfur compounds. Its empirical formula is SO 4 2-
Hydrogen Sulfate Formula
Hydrogen sulfate formula is HSO 4 . Hydrogen sulfate consists of one hydrogen atom, one sulfur atom, and four oxygen atoms. Hydrogen sulfate, also referred to as bisulfate. It typically presents as a white powder and does not have any odor. Hydrogen sulfate is a constituent of sulfuric acid, a salt with a molar mass of 97.0715 g/mol. It serves as a significant alternative to sulfur dioxide. HSO 4 stands as the chemical formula for hydrogen sulfate.
Hydrogen Sulfate Formula Charge
Hydrogen sulfate, also known as bisulfate, carries a negative charge of -1. Its chemical formula is HSO 4 -
Hydrogen Sulfate Formula Valency
The valency of hydrogen sulfate (bisulfate) is -1. This means it typically gains one electron to achieve a stable, negatively charged ion with a -1 valency. The chemical formula for hydrogen sulfate is HSO 4 - .
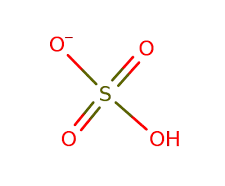
The hydrogen sulfate ion, often written as HSO 4 - , consists of one hydrogen atom (H), one sulfur atom (S), and four oxygen atoms (O). This ion carries a charge of -1, making it a negatively charged species. The structure can be illustrated as follows:
This structure shows the arrangement of the atoms in the hydrogen sulfate ion, with the sulfur atom at the center, bonded to one hydrogen atom and four oxygen atoms, resulting in the overall charge of -1.
Hydrogen Sulfate Formula Physical Properties
The molar mass of hydrogen sulfate is 97.0715 g/mol.
It hold a density of 2.345 g/cm3.
In its physical form, hydrogen sulfate is a white powder with no discernible odor.
It exhibits a complexity level of 76.
The melting point of hydrogen sulfate is 58.5°C.
Hydrogen sulfate readily dissolves in water.
The pH of hydrogen sulfate is extremely low, measuring at 1.
Hydrogen Sulfate Formula Chemical Properties
Upon its interaction with water, hydrogen sulfate generates the hydronium ion and sulfate ion as depicted by the following chemical equation:
HSO 4 – + H 2 O → H 3 O + + SO 4 2-
In the presence of the nitrate ion, hydrogen sulfate is capable of simultaneously producing nitrous acid and sulfate ion. This chemical process is represented as follows:
HSO 4 – + NO 2 – → SO 4 2- + HNO 2
Uses of Hydrogen Sulfate
Hydrogen Sulfate: Versatile Applications
Hydrogen sulfate, a chemical compound, finds diverse applications to cater to various needs. Below, you will find some of its significant applications:
It serves as a viable replacement for liquid sulfur dioxide in the removal of residual chlorine during wastewater treatment.
The sodium salt of hydrogen sulfate, known as niter cake, was traditionally produced through an outdated process.
Hydrogen sulfate resin proves effective for soothing human skin.
The reddish juice derived from hydrogen sulfate is used in styptic pencils to help staunch bleeding.
Hydrogen sulfate, with the chemical formula HSO4-, is a significant component of sulfuric acid. It has acidic properties, is a white powder with a low pH, and readily dissolves in water. It finds applications in wastewater treatment, niter cake production, skin soothing, and styptic pencils for bleeding control.
| Related Links | |
| Bromic Acid Formula | Sodium Nitride Formula |
| Cadmium Sulphate Formula | Sodium Iodide Formula |
Hydrogen Sulfate Formula FAQs
What is the formula for hydrogen sulfate?
What is the charge of the hydrogen sulfate ion?
What are the components of the hydrogen sulfate ion?
What is the valency of hydrogen sulfate (bisulfate)?
What is the molar mass of hydrogen sulfate?