
Bismuth Trichloride Formula: Bismuth, denoted by the symbol Bi and It holds an atomic number of 83. It is a dense, silvery metal with a subtle pink color. It stands out as the most diamagnetic among all metallic elements. The yellow pigment known as bismuth oxide finds widespread application in cosmetics and paints, while its compounds have been used as medicinal agents, falling within the category of antibacterial medications. Elemental bismuth itself is non-toxic. However, caution is necessary when dealing with bismuth salts, which can lead to toxic effects.
Chlorine represented by the symbol Cl and with an atomic number of 17. It exists as a corrosive greenish-yellow gas that is known for its toxicity and due to toxicity it causes irritation to both the eyes and the respiratory system. It ranks as the second lightest element within the halogen group, sandwiched between fluorine and bromine in the periodic table. Chlorine serves as an effective bleaching agent and was initially discovered by Carl Wilhelm Scheele in the year 1774.
Bismuth Trichloride Formula
Bismuth Trichloride formula is BiCl 3 , constitutes an inorganic compound with covalent characteristics. It presents as a colourless hygroscopic solid and is also recognized under the names trichloro bismuth and bismuth chloride. Bismuth Trichloride encompasses both bismuth and chloride ions within its structure and is synthesized through the reaction between bismuth and chlorine. Bismuth trichloride serves as a reliable source of bismuth and It plays an important role in the formation of various other bismuth compounds.
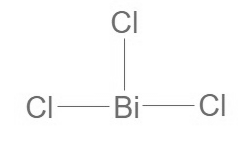
Bismuth Trichloride Formula Structure
Bismuth Trichloride formula is BiCl 3. Structural aspects of Bismuth trichloride, we can establish its Lewis structure. Bismuth is endowed with five valence electrons, while chlorine carries seven valence electrons. Considering the presence of three chlorine atoms, the cumulative valence electrons amount to 5 + 7(3) = 26. To construct the Lewis structure, bismuth is placed at the central position, and the three chlorine atoms are arranged around it. This results in a structural configuration as illustrated below.
Preparation of Bismuth Trichloride
Bismuth Trichloride can be prepared through several methods: Direct Reaction of Bismuth with Chlorine: When bismuth is exposed to chlorine, a chemical reaction occurs: 3 Cl 2 + 2 Bi → 2 BiCl 3 Reaction of Hydrochloric Acid with Bismuth Pentoxide: Bismuth Trichloride is formed when hydrochloric acid reacts with bismuth pentoxide, resulting in the production of water and chlorine as byproducts: 10 HCl + Bi 2 O 5 → 5 H 2 O + 2 Cl 2 + 2 BiCl 3 Reaction of Sodium Chloride with Bismuth Nitrate: Bismuth Trichloride is produced through the reaction of sodium chloride with bismuth nitrate and produce sodium nitrate as a byproduct: 3 NaCl + Bi(NO 3 ) 3 → 3 NaNO 3 + BiCl 3 Reaction of Hydrochloric Acid with Potassium Bismuthate: When hydrochloric acid interacts with potassium bismuthate, it results in the production of bismuth trichloride, along with water, chlorine, and potassium chloride: 6 HCl + KBiO 3 → 3 H 2 O + Cl 2 + KCl + BiCl 3 These methods provide various routes for the preparation of Bismuth Trichloride, allowing for its utilization in diverse applications.Bismuth Trichloride Formula Physical Properties
Bismuth Trichloride formula is BiCl 3.Bismuth Trichloride has a molecular weight of 315.34 gm/mol.
It has a boiling point of 447°C.
The melting point of Bismuth Trichloride ranges between 230 to 232°C.
Bismuth Trichloride Formula Chemical Properties
The chemical formula for Bismuth Trichloride is BiCl 3 .
Bismuth Trichloride can react with tin dichloride, resulting in the formation of tin tetrachloride and bismuth:
3 SnCl 2 + 2 BiCl 3 → 3 SnCl 4 + 2 Bi
When Bismuth Trichloride interacts with hydrogen sulfide, it leads to the production of hydrochloric acid and bismuth sulfide:
3 H 2 S + 2 BiCl 3 → 6 HCl + Bi 2 S 3
Tin Chloride can react with Bismuth Trichloride, giving rise to tin tetrachloride and bismuth:
BiCl 3 + SnCl → SnCl 4 + Bi
Bismuth Trichloride, when combined with nitric acid and water, forms hydrochloric acid and bismuth oxynitrate:
H 2 O + HNO 3 + BiCl 3 → 3 HCl + BiONO 3
These properties showcase the molecular, physical, and chemical characteristics of Bismuth Trichloride, highlighting its diverse reactivity in various chemical reactions.
Uses of Bismuth Trichloride
Bismuth (III) Chloride is applied in various significant ways:
It serves as a catalyst in chemical processes, such as acetolysis, hydrolysis, and alcoholysis.
Bismuth (III) Chloride is used in the preparation of thiiranes from oxiranes, contributing to the synthesis of these important compounds.
It plays an important role in the allylation of secondary benzyl alcohols, facilitating specific chemical reactions.
Health Effects
Bismuth (III) Chloride is applied in various significant ways:
It serves as a catalyst in chemical processes, such as acetolysis, hydrolysis, and alcoholysis.
It plays an important role in the allylation of secondary benzyl alcohols, facilitating specific chemical reactions.
Bismuth (III) Chloride is used in the preparation of thiiranes from oxiranes, contributing to the synthesis of these important compounds.
| Related Links | |
| Sodium peroxide Formula | Bromic Acid Formula |
| Sodium Nitride Formula | Cadmium Sulphate Formula |
Bismuth Trichloride Formula FAQs
What is the chemical formula for Bismuth Trichloride?
What is the structure of Bismuth Trichloride?
How is Bismuth Trichloride prepared?
What are the physical properties of Bismuth Trichloride?
What are some chemical properties of Bismuth Trichloride?










