
Hypophosphoric Acid Formula: Hypophosphoric acid formula is H 4 P 2 O 6 . It is characterized by a +4 oxidation state of phosphorus and It is found in a dihydrate state, H 4 P 2 O 6 .2H 2 O, as a solid. Hypophosphoric acid can be produced through the reaction of red phosphorus with sodium chlorite at room temperature.
Hypophosphoric acid, with the chemical formula H 4 P 2 O 6 , exhibits acidic properties and is characterized by the presence of phosphorus in a specific oxidation state. This compound comprises four hydrogen, two phosphorus, and six oxygen atoms. In its structure, a P-P bond exists, with each phosphorus atom forming two P-OH bonds and one P-O bond.
The P-P bond has a length of 219 picometers (pm), while the P-OH bond measures 159 pm, and the P-O bond is 151 pm long. An oxygen bridge connects one phosphorus atom to another, leading to the formation of phosphoric acid. In its solid state hypophosphoric acid is present as the dihydrate.
The chemical formula for Hypophosphoric acid is H 4 P 2 O 6 , and it typically exists as the dihydrate, H 4 P 2 O 6 .2H 2 O, also known as di-phosphoric acid
Hypophosphoric Acid Formula
The Hypophosphoric acid formula is H 4 P 2 O 6. Hypophosphoric acid consists of four hydrogen atoms, two phosphorus atoms, and six oxygen atoms. In its molecular structure is a P-P bond, and each phosphorus atom forms two (P-OH) bonds and one (P-O) bond. The P-P bond stretches over 219 picometers (pm), the P-OH bond extends to 159 pm, and the P-O bond measures 151 pm.
Hypophosphoric Acid Formula Structure
The Hypophosphoric acid formula is H 4 P 2 O 6 . Its structure consists of two phosphorus atoms, connected by a P-P bond, with each phosphorus atom bonded to two hydroxyl (OH) groups and one oxygen atom (O).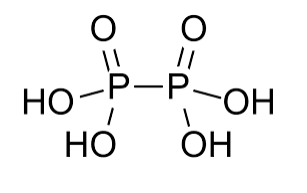
Hypophosphoric Acid Oxidation State
The oxidation state of phosphorus in Hypophosphoric acid (H 4 P 2 O 6 ) is +5.Hypophosphoric Acid Basicity
The H 4 P 2 O 6 structure features four P-OH bonds, which results in four acidic hydrogens. Consequently, the basicity of hypophosphoric acid is 4.Hypophosphoric Acid Preparation
Hypophosphoric acid can be produced by combining red phosphorus with sodium chlorite at room temperature. Alternatively, when white phosphorus is partially dissolved in water. It undergoes oxidation in the presence of air. resulting in a mixture of Hypophosphoric acid, phosphorous acid, and phosphoric acid. The chemical equation for this reaction is:
2P + 4NaOCl 2 + 2H 2 O → H 4 P 2 O 6 + 2NaClThere is no P-H bonds, which means it cannot work as a reducing agent. It has four acidic hydrogens and includes a P-P bond in its structure.
Hypophosphoric Acid Formula Properties
Hypophosphoric acid is a white solid that exists in a dihydrate state. It is odorless. It has a melting point of 54°C. This acid is soluble in water. Its molar mass is 161.98 g/mol in the form of a white crystalline powder.
Hypophosphoric Acid Properties.
- Common Name: Hypophosphoric Acid
- Alternate Name: Diphosphoric Acid
- Physical State: White Solid
- Formula: H4P2O6
- Melting Point: 54 °C
- Molar Mass: 161.98 g/mol
- Water Solubility: Soluble
Health Hazards of Hypophosphoric Acid
Hypophosphoric acid is very corrosive and can cause burns on the skin and eyes. Breathing it in can irritate the lungs, and it can also irritate the eyes and eyelids. If it gets on your skin or in your eyes, wash it off immediately with water. Waiting can cause lasting eye damage. Don't swallow it, as it can hurt your mouth, throat, esophagus, and stomach..
Uses of Hypophosphoric Acid
Hypophosphoric acid finds diverse utility in various fields. It serves as a bleaching agent. Additionally, it exhibits excellent wetting properties. In the pharmaceutical sector it is used as a stimulant. Furthermore, its chemical versatility is evident through its use as a reducing agent, a tetrabasic acid, and a wetting agent in numerous chemical applications.
| Related Links | |
| Selenous Acid Formula | Pyrophosphoric Acid Formula |
| Potassium Sulfite Formula | Dinitrogen Pentoxide Formula |
Hypophosphoric Acid Formula FAQs
What is the chemical formula of hypophosphoric acid?
What is the oxidation state of phosphorus in hypophosphoric acid?
How is hypophosphoric acid prepared?
What is the basicity of hypophosphoric acid?
What is the physical state of hypophosphoric acid?










