
Magnesium Chloride Formula: Magnesium Chloride, also known as Magnesium Dichloride or Chloromagnesite, is a chemical compound represented by the molecular formula MgCl 2 .This ionic salt exhibits high solubility in water, comprising one magnesium ion (Mg +2 ) and two chloride ions (Cl – ). Magnesium chloride can exist in either hydrated or anhydrous crystal forms and can be sourced from seawater or brine solutions. This naturally occurring inorganic compound, essential for human health, finds diverse applications in both industry and medicine.
Also Check - Lithium Bromide Formula
Chemical Formula of Magnesium Chloride
Magnesium, with a charge of +2, tends to lose two electrons in its reaction with chlorine, which has a charge of -1 and readily accepts one electron. To achieve a balanced electron configuration, two chlorine atoms are required to accept magnesium's two electrons, resulting in the chemical formula MgCl 2 .
Also Check - Hydrogen Formula
Structure of Magnesium Chloride
Magnesium chloride crystallises in the cadmium chloride structure, featuring an octahedral magnesium atom at its core. This compound forms through ionic bonding between one Mg +2 ion and two Cl -1 ions. The presence of lone pairs of electrons on magnesium causes a slight deviation from a perfect 180-degree angle, resulting in a bent structure. Various hydrated forms of magnesium chloride are based on the water of crystallisation within the molecule.
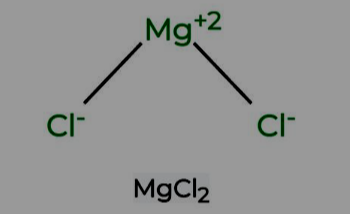
Formation of Magnesium Chloride
In the formation of magnesium chloride, magnesium, with an atomic number of 12 and two electrons in its outermost shell, donates two of these electrons to two chlorine atoms, each with an atomic number of 17. This electron-sharing process results in the creation of magnesium ions and chloride ions, linked by ionic or electrovalent bonds.

Preparation of Magnesium Chloride
Magnesium chloride (MgCl 2 ) can be naturally occurring or prepared through several methods. One common approach involves the reaction of magnesium with hydrochloric acid, yielding MgCl 2 and hydrogen gas. Additionally, magnesium chloride can be formed when magnesium carbonate reacts with HCl, producing MgCl 2 along with carbon dioxide and water. The Dow process is another method for its production. Anhydrous magnesium chloride can also be obtained by reacting magnesium with mercury (II) chloride.
When magnesium (Mg) undergoes a reaction with hydrochloric acid (HCl), it results in the formation of magnesium chloride (MgCl 2 ) along with the release of hydrogen gas, as depicted by the equation:
Mg + 2HCl → MgCl 2 + H 2
Another common method of producing magnesium chloride is by reacting magnesium carbonate (MgCO 3 ) with HCl, yielding magnesium chloride, carbon dioxide (CO 2 ), and water (H 2 O):
MgCO 3 + 2HCl → MgCl 2 + CO 2 + H 2 O
The Dow process is a widely used industrial method for the synthesis of magnesium chloride. It typically involves the reaction between magnesium and calcium hydroxide (Ca(OH)2), leading to the formation of magnesium hydroxide (Mg(OH)2) and calcium ions (Ca+2):
Mg +2 + Ca(OH) 2 → Mg(OH) 2 + Ca +2
Subsequently, magnesium hydroxide can be further converted to magnesium chloride by reacting it with hydrochloric acid:
Mg(OH) 2 + 2HCl → MgCl 2 + H 2 O
Additionally, anhydrous magnesium chloride can be prepared through a chemical reaction between magnesium and mercury (II) chloride (HgCl 2 ), resulting in magnesium chloride and mercury (Hg):
Mg + HgCl 2 → MgCl 2 + Hg
Also Check - Hydrocarbons Formula
Properties of Magnesium Chloride
Physical Properties
- It appears as a white or colourless crystalline solid.
- It is odourless.
- The molecular weight of magnesium chloride is 95 g/mol.
- The density of anhydrous magnesium chloride is 2.32 g/cm³.
- It has a melting point of 714°C and a boiling point of 1412°C.
- It is highly soluble in water and partially soluble in non-polar solvents.
Chemical Properties
- Upon heating, it gradually loses its water of crystallization.
- It is prepared by reacting magnesium hydroxide with hydrochloric acid.
- When added to water, it disintegrates into magnesium and chloride ions.
- It reacts with bases to form magnesium hydroxide and a salt.
Also Check - Alcohol Phenol and Ether
Applications of Magnesium Chloride
Magnesium Metal Production: Magnesium chloride is a key component in the production of magnesium metal, vital for various industries.
Fire Extinguishers: It acts as a catalyst in fire extinguishers, creating a non-flammable magnesium oxide layer, enhancing fire suppression.
Food Processing: Magnesium chloride serves as a coagulating agent in food production, crucial for tofu and soy-based products.
Catalyst in Chemistry: It enhances the speed of chemical reactions, acting as a catalyst in a variety of scientific and industrial procedures.
Disinfectants: Magnesium chloride is essential in disinfectant formulations, improving their effectiveness against microorganisms.
Thread Lubricant: It functions as a reliable thread lubricant in machinery, reducing friction and extending equipment lifespan.
Also Check - Amine Formula
Magnesium Chloride, denoted by the molecular formula MgCl 2 , is a versatile chemical compound of paramount significance across multiple domains. Its distinctive ionic characteristics, crystalline structure, and exceptional properties render it invaluable in a wide array of practical applications.
From its formation through electron sharing between magnesium and chlorine to its preparation methods, including reactions with hydrochloric acid or magnesium carbonate, we have explored the fundamental aspects of this compound.
Key physical properties, such as its crystalline appearance, density, and solubility, as well as chemical properties like its reaction with bases and behaviour upon heating, further define its characteristics.
Overall, Magnesium Chloride's significance in both industrial processes and essential products underscores its pivotal role in chemistry, technology, and various sectors, making it a compound of enduring relevance and utility.
Magnesium Chloride Formula FAQs
What is the chemical formula for magnesium chloride?
How is magnesium chloride commonly produced?
What is the Dow process?
What happens when magnesium reacts with hydrochloric acid?










