
Mercury II Nitrate Formula : Mercury, also known as quicksilver, is a naturally occurring element that can be found in rocks and coal deposits. The name "mercury" is derived from the Greek words "hydrargyrum," which mean "water" and "silver." Mercury is represented by the chemical symbol Hg and has an atomic number of 80. It is a unique liquid metal classified under group 12 in the periodic table, possessing an atomic mass of 200.59 atomic mass units (u) and a low melting point of -38.83 degrees Celsius.
Nitrogen is a vital element for all living organisms. When nitrogen combines with oxygen, it naturally forms nitrate compounds. Most inorganic nitrates are soluble in water. Nitrogen has a molar mass of approximately 62.0049 grams per mole (g/mol). It exists as a polyatomic ion with the chemical formula NO3– and is widely used in the production of fertilizers and pharmaceutical drugs.
Mercury II Nitrate
Mercury II Nitrate, also known as Mercuric Nitrate or Mercury dinitrate, is a hazardous chemical compound. It is sometimes used as a reagent in laboratories, but caution is required when handling it. This substance appears as a white crystalline solid and can easily dissolve in water. Its density is approximately 4.3 grams per cubic centimeter (g/cm³).
However, it's important to be aware that when exposed to high temperatures for an extended period, Mercury II Nitrate can become explosive and release harmful nitrogen oxides when heated. So, it's essential to follow safety rules when dealing with this substance because of its high toxicity, and many countries have rules for its use.
Mercury II Nitrate Formula
The Mercury II Nitrate Formula is Hg(NO 3 ) 2 . The "Hg" symbol represents mercury, and "NO 3 " represents nitrate. It has a molar mass of 324.7 grams per mole (g/mol).Mercury II Nitrate Formula Structure
The chemical structure of Mercury(II) Nitrate (Hg(NO 3 ) 2 ) can be represented as follows:
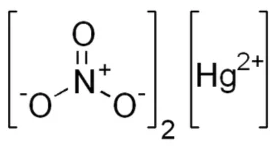
In this structure, "Hg" represents the mercury atom, and "NO3" represents the nitrate ion, consisting of one nitrogen atom (N) bonded to three oxygen atoms (O). Mercury(II) Nitrate contains two of these nitrate ions, resulting in the chemical formula Hg(NO 3 ) 2 .
Mercury II Nitrate Formula Physical Properties
Here are some of the physical properties of Mercury II Nitrate:
- Melting point: 79 degrees Celsius
- Soluble in water
- Density: 4.3 g/cm³
- Insoluble in alcohol but soluble in nitric acid, acetone, and ammonia
- Corrosive to many metals
- Molar mass: 324.7 g/mol
- Non-flammable
Mercury II Nitrate Formula Chemical Properties
Mercury (II) Nitrate exhibits distinct chemical properties:Hydrolysis: When exposed to water or an alkaline solution, Mercury (II) Nitrate can easily undergo hydrolysis, leading to the formation of Mercury(II) Oxide (HgO) and the release of nitric acid (HNO 3 ):
Hg(NO 3 ) 2 + H 2 O → HgO + 2HNO 3Formation of Metallic Mercury: To obtain metallic mercury from the nitrate compound, you can heat Mercury (II) Nitrate using a distillation apparatus. At temperatures exceeding 400°C, it readily decomposes, yielding metallic mercury (Hg), nitrogen dioxide (NO 2 ), and oxygen (O 2 ):
Hg (NO 3 ) 2 → Hg + 2NO 2 + O 2Hazards and Safety Measures
Mercury (II) Nitrate presents significant hazards, and safety measures are essential:
High Toxicity: This compound is highly toxic and can cause severe health problems when there is extensive exposure.
Explosive When Heated: If subjected to heat, mercury (II) nitrate can lead to explosions.
Safety Measures
When working with mercury (II) nitrate, proper safety precautions must be followed, and direct contact should be avoided.
Protective Clothing: Utilize appropriate protective clothing to prevent skin contact and inhalation of fumes or dust.
Uses of Mercury (II) Nitrate Formula
Mercury (II) Nitrate, also known as mercuric nitrate, has several applications:
Preparation of Mercury Fulminate: It is used in making mercury fulminate, a sensitive explosive compound.
Mercuration Reactions: It is used in chemical reactions involving the addition of mercury to organic compounds (mercuration).
Analytical Reagents: In laboratories, mercuric nitrate serves as an analytical reagent for various tests and experiments. .
Ointments: Mercuric nitrate can be found in certain ointments for medicinal purposes.
| Related Link | |
| Hydrobromous Acid Formula | Hyposulfurous Acid Formula |
| Iodide Formula | Molybdic Acid Formula |
Mercury II Nitrate Formula FAQs
What is the chemical formula for Mercury II Nitrate?
What does "Hg" represent in the formula?
What is the molar mass of Mercury II Nitrate?
Is Mercury II Nitrate soluble in water?
What is the melting point of Mercury II Nitrate?










