

Metallurgy is the branch of science and technology concerned with the properties of metals and their production and purification. The metallurgical process can be divided into a few general steps:
Mining: Extraction of the ore from the earth.
Occurrence of Ores
Metallic ores are minerals or rocks from which metals can be economically extracted. They occur in various forms:
Native Form: Some metals occur in the pure state, e.g., gold, silver, platinum. Oxides:
Example:
Hematite - Fe 2 O 3
Bauxite - Al 2 O 3 .2H 2 O
Sulfides:
Example: Galena- PbS
Chalcopyrite - CuFeS 2
Carbonates:
Example: Calcite - CaCO 3
Dolomite - CaMg(CO 3 ) 2
Halides:
Example: Rock salt - NaCl
Fluorspar - CaF 2
Concentration of Ores
The aim of ore concentration is to enrich the ore, eliminating unwanted rocky matter.
A. Hydraulic Washing (Gravity Separation): This method is based on the differences in the weights (specific gravities) of the ore and gangue particles.
Procedure: The powdered ore is washed with a jet of water. The lighter gangue particles are washed away, while the heavier ore particles settle down and can be separated.
B. Magnetic Separation: This method is based on the differences in magnetic properties of the ore and the impurities.
Procedure: The powdered ore is placed on a conveyor belt, which moves past a magnet. Magnetic components stick to the magnet while non-magnetic components fall off the belt.
Example: Separation of wolframite ( FeWO 4 ) from cassiterite (SnO 2 ) , where wolframite is magnetic while cassiterite is non-magnetic.
C. Leaching: Leaching is the process of extracting a substance from a solid by dissolving it in a liquid. In the context of metallurgy, leaching is used to extract metals from their ores using a suitable solvent.
Examples: Silver: Silver is leached from its ore using sodium cyanide solution.
Ag 2 S + 4NaCN → 2Na[Ag(CN) 2 + Na 2 S
Aluminium: Bauxite is treated with sodium hydroxide solution, which dissolves aluminum oxide producing sodium aluminate, leaving impurities behind.
Al 2 O 3 + 2NaOH → 2NaAlO 2 + H 2 O
In all these processes, after concentration, the concentrated ore is subjected to further processes like reduction (to obtain the metal) and refining.
Also Check – Percent by Weight Formula
Extraction of Crude Metal from Concentrated Ores
The extraction primarily involves two processes:
Reduction: It involves the removal of oxygen, sulfur, etc., from the ore.
Oxidation: It involves the addition of oxygen or removal of hydrogen.
A. Pyrometallurgy (Using Heat): For metals like Zinc, Fe, and Pb.
ZnO +C → Zn + CO
B. Hydrometallurgy (Using Aqueous Solutions): Especially for metals like Au, and Ag.
4Au + 8NaCN + O 2 + 2H 2 O → 4Na[Au(CN) 2 ]+4NaOH
C. Electrometallurgy (Electrolytic Reduction): Used for reactive metals like Na, K, Mg, Al, etc.
Al 2 O 3 → 2Al + 3O 2
Highly Reactive Metals:
These metals, such as sodium, potassium, calcium, etc., are found in nature as their oxides or chlorides. Extraction is typically done through electrolytic reduction.
Example:
Extraction of sodium:
NaCl (l) → Na (l) + Cl 2(g)
The above reaction is carried out in a Downs cell, where molten sodium chloride is electrolytically reduced to produce sodium metal and chlorine gas.
Moderately Reactive Metals :
Metals like zinc, iron, lead, etc., are found as oxides, sulfides, or carbonates in nature. These metals are usually reduced by using carbon or carbon monoxide.
Examples:
A. Extraction of zinc:
ZnO(s) + C(s) → Zn(s) + CO(g)
B. Extraction of iron in a blast furnace:
Fe 2 O 3(s) + 3CO (g) → 2Fe (l) + 3CO 2(g)
Least Reactive Metals:
These metals, such as gold, silver, platinum, etc., are often found in the free state in nature due to their low reactivity. They can be extracted from their ores by simple methods like heating with a suitable reducing agent. Example:
Extraction of silver from its ore using sodium cyanide:
Ag 2 S (s) + 4NaCN (aq) → 2Na[Ag(CN) 2 ] (aq) +Na 2 S (aq)
Followed by:
Na[Ag(CN) 2 ] (aq) + Zn (s) → Ag (s) + Na[Zn(CN) 4 ] (aq)
Also Check – Percentage Yield Formula
Thermodynamic Principles of Metallurgy
The reduction process should be thermodynamically feasible. The Gibbs free energy change, ΔG, should be negative for the process.
The formula is: ΔG=ΔH−TΔS
Where:
ΔH is enthalpy change
ΔS is entropy change
T is temperature in Kelvin
Also Check – Gibbs Free Energy Formula
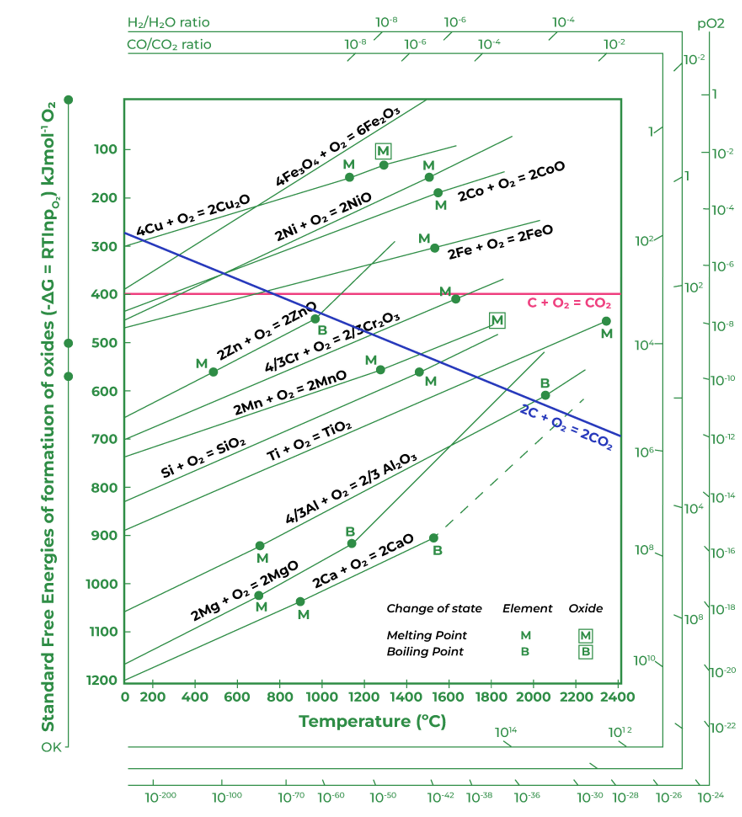
Ellingham Diagram
It's a graphical representation showing the variation of standard free energy change of formation of oxides with temperature.
Key points: The more negative the ΔG, the greater the stability of the oxide. Lines for metals which are found native (like gold) aren't present because their oxides aren't stable. Lines of metal oxides that intersect are of significance. The metal represented by the upper line can reduce the oxide of the metal represented by the lower line.
Electrochemical Principles of Metallurgy
The metal ores which are not very reactive can be extracted by electrochemical methods. The underlying principle is that reduction of cations to metal at the cathode:
M n+ + n e− →M
Example:
Extraction of Aluminum:
Aluminum is extracted from purified alumina (Al 2 O 3 ) dissolved in molten cryolite (Na 3 AlF 6 ) by the Hall-Heroult process.
At cathode: Al 3+ + 3e − → Al
At anode: 2O 2− →O 2 + 4e –
Oxidation-Reduction: In metallurgy, roasting is an oxidation process, and smelting is a reduction process.
Example:
Extraction of Iron :
2Fe 2 O 3 + 3C → 4Fe + 3CO 2
Refining Techniques
After extraction, metals are purified using various techniques:
- Distillation: For metals with low boiling points, like Zn and Hg.
- Liquation: If the metal is easily fusible, it's heated on a sloping hearth. The molten metal flows away, leaving behind the impurities.
- Electrolytic Refining: Purification of copper. Impure copper is made the anode, and pure copper is the cathode. On passing electricity, copper dissolves from the anode and deposits at the cathode.
- Zone Refining: Used for ultra-pure metals. A circular mobile heater moves over an impure metal rod. The impurities move to the next molten zone.
- Vapour Phase Refining: The metal is converted to its volatile compound and then decomposed to get pure metal.
Example: Mond's process for nickel refining.
4 Ni + 4CO→Ni(CO) 4
Ni(CO) 4 → Ni + 4CO
Metallurgy Formula FAQs
Q1. What is froth flotation?
Q2. What is leaching?
Q3. What is an alloy?
Q4. What is a blast furnace used for?
Q5. What is the role of limestone in a blast furnace?











