Matter In Our Surroundings Class 9 NCERT Solutions help students understand the very first and most fundamental chapter of Class 9 Science.
Chapter 1 – Matter in Our Surroundings introduces the concept of matter, its physical nature, states, and how it behaves under different conditions. This chapter forms the base for many topics studied later in chemistry and physics.
The Matter In Our Surroundings Class 9 Question Answers are explained in a clear and step-by-step manner, making them easy to understand for beginners. These solutions strictly follow the NCERT textbook and are ideal for exam preparation, homework help, and quick revision for 9th Class Science Ch 1.
What is Matter in Our Surroundings?
Matter is anything that occupies space and has mass. Everything around us, such as air, water, food, and objects, is made up of matter. According to 9th Class Science Chapter 1, matter is composed of very small particles that are constantly moving and attract each other. These basic ideas help students understand the physical nature of matter.
Matter exists in three main states—solid, liquid, and gas. Each state has different properties based on how closely the particles are packed and how they move. The chapter also introduces how matter changes its state due to changes in temperature and pressure, such as melting, evaporation, and condensation, using simple day-to-day examples.
Matter In Our Surroundings Class 9 Exercise NCERT Solutions
The Matter In Our Surroundings Class 9 Exercise solutions provide clear answers to all textbook questions. Each answer is written in simple language and includes proper explanations, examples, and definitions where required.
These 9th Science Chapter 1 Question Answer solutions are especially useful for revision before unit tests and annual exams.
CBSE Class 9th Science Chapter 1 Question Answers
Question 1. Which of the following are matter? Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.Question 3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Question 5. The mass per unit volume of a substance is called density. (density = mass/volume). Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
| Differences in the characteristics of states of matter | |||
|
S. No . |
Solid state |
Liquid state |
Gaseous state |
|
1. |
Definite shape and volume. | No definite shape. Liquids attain the shape of the vessel in which they are kept. | Gases have neither a definite shape nor a definite volume. |
|
2. |
Incompressible | Compressible to a small extent. | Highly compressible |
|
3. |
There is little space between the particles of a solid. | These particles have a greater space between them. | The space between gas particles is the greatest. |
|
4. |
These particles attract each other very strongly. | The force of attraction between liquid particles is less than solid particles. | The force of attraction is least between gaseous particles. |
|
5. |
Particles of solid cannot move freely. | These particles move freely. | Gaseous particles are in a continuous, random motion. |
(b) Rigidity: It is the property of matter to maintain its shape even if external forces work and the solids show this property.
Compressibility: It is the property of matter to allow compression under high pressure and the gases show this property. Fluidity: It is the property of a substance to easily flow and allow change in its shape under external forces and this property is exhibited by both liquids and gases.Filling a gas container: Gases can be compressed easily hence they can be filled within a vessel at high pressure. This property of gases allows their convenient filling into a small container or cylinder and that also in a large volume. It also allows their easy transport from one place to the other e.g. CNG.
Shape: According to the type of matter shape differs depending upon location of particles like Solids have definite shape while Liquids acquire the shape of their container and gases as such don’t have any shape.Kinetic energy: It is the kind of energy present in an object when it is under motion as the particles of that object/matter are continuously moving therefore matter has kinetic energy. However greater is the movement more will be the kinetic energy and vice a versa i.e. solid < liquid < gas
Density: Mass per unit volume of a substance/matter is known as its density i.e. density = mass/volume Question 7. Give reasons:(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Solution:(a) Since the attraction force between particles of a gas is negligible i.e. extremely less hence particles freely move/flow in all possible directions as a result gas fills completely the vessel in which it is kept.
(b) Freely moving particles of gas hit the walls of its container continuously and randomly therefore such random and erratic motion of gas particles exerts pressure on the walls of the container.
(c) A wooden table particle is quite rigid, have a fixed location and also possess a definite shape and volume. Due to all these properties, we should call a wooden table a solid substance.
(d) Air is a mixture of gases and since particles of gas are far apart so same is true for air therefore, we can easily move our hand in air. But a solid block of wood is hard and rigid that resists any change in location of its particles hence we need a karate expert in case of a solid block of wood.
Question 8. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why?Solution: (a) 300 K = (300 − 273)°C = 27°C (b)573 K = (573 − 273)°C = 300°C
Question 10. What is the physical state of water at: (a) 250°C (b) 100°C
Solution: (a) Water at 250°C exists in gaseous state. (b) At 100°C, water can exist in both liquid and gaseous form. At this temperature, after getting the heat equal to the latent heat of vaporization, water starts changing from liquid state to gaseous state.
Question 11. For any substance, why does the temperature remain constant during the change of state?
Solution: During a change of state, the temperature remains constant. This is because all the heat supplied to increase the temperature is utilised in changing the state by overcoming the forces of attraction between the particles. This heat is called the latent heat. Latent heat does not contribute in increasing the temperature of the substance.
Question 12. Suggest a method to liquefy atmospheric gases.
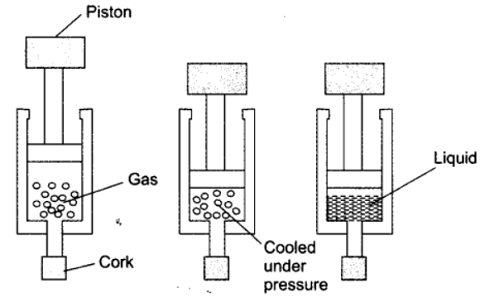
Question 13. Why does a desert cooler cool better on a hot dry day?
Solution: When a liquid evaporates, the particles of the liquid absorb energy from the surroundings to compensate the loss of energy during evaporation. This makes the surroundings cool. In a desert cooler, the water inside it is made to evaporate. This leads to absorption of energy from the surroundings, thereby cooling the surroundings. Again, we know that evaporation depends on the amount of water vapour present in air (humidity). If the amount of water vapour present in air is less, then evaporation is more. On a hot dry day, the amount of water vapour present in air is less. Thus, water present inside the desert cooler evaporates more, thereby cooling the surroundings more. That is why a desert cooler cools better on a hot dry day.
Question 14. How does the water kept in an earthen pot (matka) become cool during summer?Solution: The earthen pot is porous with lot of pores on it, the water oozes out through these pores and the water gets evaporated at the surface of the pot thereby causing cooling effect. This makes the pot cold and the water inside the pot cools by this process.
Question 15. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Solution: Acetone, petrol or perfume evaporate when they come into contact with air. The evaporation causes cooling sensation in our hands.
Solution: Saucer has a bigger surface area as compared to cup. Since evaporation is a surface phenomenon, by using a saucer instead of cup we are increasing the surface are for evaporation to occur. Faster evaporation of particles of tea or milk allows cooling and taking a sip becomes easier.
Question 17. What type of clothes should we wear in summer?
Class 9 Science Matter In Our Surroundings Extra Questions
1. Convert the following temperature to Celsius scale.
(a) 293K (b) 470K
Solution:
0°C=273K
(a) 293K= (293 – 273)°C = 20°C
(b) 470K= (470 – 273)°C = 197°C
2.Convert the following temperatures to the Kelvin scale.
(a) 25°C (b) 373°C
Solution:
0°C = 273K
(a) 25°C = (25+273)K = 298K
(b) 373°C = (373+273)K = 646K
3. Give reason for the following observations:
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume while sitting several metres away.
Solution:
(a) At room temperature, naphthalene balls undergo sublimation wherein they directly get converted from a solid to a gaseous state without having to undergo the intermediate state, i.e., the liquid state.
(b) Molecules of air move at a higher speed and have large intermolecular spaces. Perfumes comprise substances that are volatile, which scatter quickly in air, becoming less concentrated over a distance. Hence, we are able to smell perfume sitting several metres away.
4. Arrange the following in increasing order of forces of attraction between the particles – water, sugar, oxygen.
Solution:
Oxygen (gas) < water (liquid) < sugar (solid)
5. What is the physical state of water at –
(a) 25°C (b) 0°C (c) 100°C?
Solution:
(a) At 25°C, the water will be in liquid form (normal room temperature)
(b) At 0°C, the water is at its freezing point, hence both solid and liquid phases are observed.
(c) At 100°C, the water is at its boiling point, hence both liquid and gaseous states of water (water vapour) are observed.
6. Give two reasons to justify –
(a) Water at room temperature is a liquid.
(b) An iron almirah is a solid at room temperature.
Solution:
(a) Water persists as a liquid at room temperature since its melting point is lower than room temperature and its boiling point (100o C) is higher.
Similarly,
(i). A fixed volume is occupied by a fixed mass of water.
(ii). At room temperature, water does not have a fixed shape and flows to fit the container’s shape.
As a result, water is a liquid at room temperature.
(b) Because its melting and boiling points are above room temperature, an iron almirah is a solid at room temperature. In the same way,
(i) An iron almirah is rigid and has a predetermined shape.
(ii) Metals have a relatively high density.
As a result, at room temperature, iron almirah is a solid.
7. Why is ice at 273K more effective in cooling than water at the same temperature?
Solution:
At 273 K, ice will absorb heat energy or latent heat from the medium to overcome fusion and transform into water. As a result, ice has a greater cooling impact than water at the same temperature since water does not absorb the excess heat from the medium.
8. What produces more severe burns, boiling water or steam?
Solution:
Steam produces severe burns. It is because it is an exothermic reaction that releases a high amount of heat which it had consumed during vaporization.
9. Name A, B, C, D, E and F in the following diagram showing a change in its state.
Solution:
Interconversion of three states of matter: Using temperature or pressure, any state of matter can be turned into another.
(A) Solid to Liquid → Melting (or) fusion (or) liquefaction
(B) Liquid to Gas → Evaporation (or) vaporization
(C) Gas to liquid → Condensation
(D) Liquid to Solid → Solidification
(E) Solid to Gas → Sublimation
(F) Gas to Solid → solidification
