
Specific Heat Capacity Formula: Specific heat capacity of a substance signifies the heat needed to increase the temperature of one unit mass of the substance by one degree, indicating its resistance to temperature changes, akin to thermal inertia. This formula is commonly presented with the symbol Q representing heat quantity.
Specific heat capacity, when considered in relation to heat capacity, can be expressed as follows:
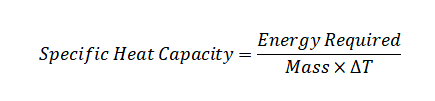
Where:
c represents the specific heat capacity.
Q represents the heat quantity.
m signifies the substance's mass.
ΔT represents the change in temperature.
Also Check - Gibbs Free Energy Formula
Specific Heat Capacity Formula Solved Examples
Example 1: A 125-gram piece of copper has a heat capacity of 19687.6 joules and is heated from 150°C to 250°C. Calculate the specific heat capacity of copper.
Solution:
Given:
Mass (m) = 125 grams
Heat capacity (Q) = 19687.6 joules
Change in temperature (ΔT) = 250°C - 150°C = 100°C
To find the specific heat (c), we can use the formula:
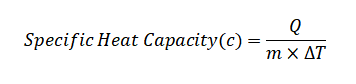
Substituting the given values:
c = 19687.6 J / (125 g * 100°C)
c = 1.575 J/g°C
Also Check - Percentage Yield Formula
Example 2: A 250-gram aluminum block initially at 20°C is heated, and it absorbs 7500 joules of heat energy. As a result, its temperature increases to 75°C. Calculate the specific heat capacity of aluminium.
Solution:
Given:
Mass (m) = 250 grams
Initial temperature (T1) = 20°C
Final temperature (T2) = 75°C
Heat absorbed (Q) = 7500 joules
First, calculate the change in temperature (ΔT):
ΔT = T2 - T1
ΔT = 75°C - 20°C
ΔT = 55°C
Now, we can calculate the specific heat capacity (c) using the formula:
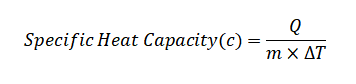
c = 7500 J / (250 g * 55°C)
c = 0.545 J/g°C
So, the specific heat capacity of aluminum is approximately 0.545 J/g°C.
Also Check - Percent by Weight Formula
Example 3: A 50-gram sample of water initially at 25°C is heated until it reaches a temperature of 75°C. During this process, 4180 joules of heat energy are supplied. Determine the specific heat capacity of water.
Solution:
Given:
Mass (m) = 50 grams
Initial temperature (T1) = 25°C
Final temperature (T2) = 75°C
Heat supplied (Q) = 4180 joules
Calculate the change in temperature (ΔT):
ΔT = T2 - T1
ΔT = 75°C - 25°C
ΔT = 50°C
Now, use the specific heat formula to find c:
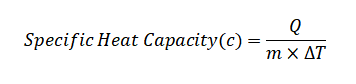
c = 4180 J / (50 g * 50°C)
c = 1.672 J/g°C
The specific heat capacity of water is approximately 1.672 J/g°C.
Also Check - Partial Pressure Formula
Example 4: A 300-gram piece of lead initially at 25°C is heated until it reaches a temperature of 200°C. During this process, 7500 joules of heat energy are supplied. Determine the specific heat capacity of lead.
Solution:
Given:
Mass (m) = 300 grams
Initial temperature (T1) = 25°C
Final temperature (T2) = 200°C
Heat supplied (Q) = 7500 joules
Calculate the change in temperature (ΔT):
ΔT = T2 - T1
ΔT = 200°C - 25°C
ΔT = 175°C
Now, use the specific heat formula to find c:
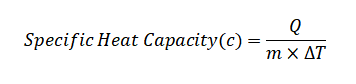
c = 7500 J / (300 g * 175°C)
c = 0.1429 J/g°C
The specific heat capacity of lead is approximately 0.1429 J/g°C.
Example 5: Find the specific heat capacity of ethanol when a 150-gram sample initially at -10°C is heated to 80°C, absorbing 5250 joules of heat energy during the process.
Solution:
Given:
Mass (m) = 150 grams
Initial temperature (T1) = -10°C
Final temperature (T2) = 80°C
Heat supplied (Q) = 5250 joules
Calculate the change in temperature (ΔT):
ΔT = T2 - T1
ΔT = 80°C - (-10°C)
ΔT = 90°C
Now, use the specific heat formula to find c:
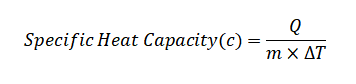
c = 5250 J / (150 g * 90°C)
c = 0.389 J/g°C
The specific heat capacity of ethanol is approximately 0.389 J/g°C.
In these examples, we calculated the specific heat capacity of different substances (aluminum and water) by using the formula and provided detailed step-by-step solutions. Specific heat capacity measures how much energy is required to change the temperature of a substance, and it varies from one substance to another.
Specific Heat Capacity Formula FAQs
What is the specific heat capacity?
Why is specific heat capacity important?
What are the units of specific heat capacity?
Is specific heat capacity constant for all substances?
What is the formula for calculating specific heat capacity?










