
Strontium Nitrate Formula: Strontium is a naturally occurring, reactive metal represented by the chemical symbol Sr. This element exhibits a silvery appearance with a subtle yellowish hue. Strontium bears a striking resemblance to calcium and is thus incorporated into bone structures. However, it's crucial to differentiate between synthetic and natural strontium. Synthetic strontium is radioactive and poses a risk to human health, whereas its naturally occurring counterpart is stable and safe.
During the 19th century, strontium found its primary application in sugar production.
Nitrate, an inorganic compound, consists of one nitrogen (N) atom and three oxygen (O) atoms, with a chemical formula of NO 3 – . Nitrate serves as a fundamental ingredient in both fertilizers and explosives, and it also finds use as a food additive.
Nitrogen, a nonmetallic chemical element with an atomic number of 7, stands as one of the most abundant elements in the universe. Approximately 3% of the human body's mass comprises nitrogen, with its primary utility lying in fertilizer production and agriculture. Additionally, it plays a significant role in explosive manufacturing.
Oxygen, a highly reactive nonmetal, bears the atomic number 8. It exists as a colorless and odorless gas and ranks as the most abundant element on Earth. Oxygen boasts various applications, including in the textile and plastic industries, as well as in submarines and space flights.
Also Check – Ammonium Nitrate Formula
Strontium Nitrate Formula
Strontium nitrate is a chemical compound consisting of strontium, nitrogen, and oxygen, and its molecular formula is Sr(NO 3 ) 2 . This solid substance is characterized by its white crystalline structure and lacks any noticeable odor. Its primary applications lie in the field of pyrotechnics, where it serves as both a red colorant and an oxidizer for fireworks and crackers.
In terms of its chemical composition, strontium carries a 2+ ionic charge (Sr +2 ), while nitrate (NO 3 ) 1- bears a 1– ionic charge. To achieve a balanced formula, two nitrate ions are needed, resulting in the formula Sr(NO 3 ) 2 .
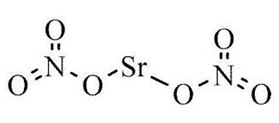
Regarding its molecular properties, strontium nitrate has a molar mass of 211.63 g/mol.
Also Check – Chemistry Formula For Solubility Product
Physical Properties of Strontium Nitrate
- Molecular Formula: Sr(NO 3 ) 2
- Appearance: White crystalline solid
- Odor: Odorless
- Solubility: It is soluble in both water and ammonia.
- Melting Point: 570°C
- Boiling Point: 645°C
- Density: 2.99 g/cm3
- Molar Mass: 211.63 g/mol
Chemical Properties of Strontium Nitrate
Aqueous strontium nitrate solution reacts with sodium sulfate, resulting in the formation of sodium nitrate and strontium sulfate as a precipitate.
Equation: Sr(NO 3 ) 2 (aq) + Na 2 SO 4 (aq) → SrSO 4 (s) + 2NaNO 3 (aq)
When strontium nitrate reacts with ammonium sulfate, it produces a white precipitate, namely strontium sulfate.
Equation: (NH4) 2 SO 4 (aq) + Sr(NO 3 ) 2 (aq) → SrSO 4 (s) + 2NH 4 NO 3 (aq)
Uses and Applications of Strontium Nitrate
Strontium nitrate finds various practical applications, including:
- Pyrotechnics: It is commonly used as an oxidizer and a red colorant in fireworks and flares.
- Signal Flares: Strontium nitrate imparts a red color to signal flares, making them visible from a distance.
- Electronics: It is utilized in the manufacturing of cathode ray tubes (CRTs) for color television screens.
- Metallurgy: In the refining of zinc and other metals, strontium nitrate can act as a desulfurizing agent.
- Photography: It was previously employed in certain types of photographic processes, although this use has declined with digital technology.
These applications showcase the versatility and importance of strontium nitrate in various industries.
Also Check – Chemical Bonding Formula
Strontium nitrate plays a vital role in various applications, including its use in pyrotechnics for its red coloration and oxidizing properties, as well as in electronics for cathode ray tube manufacturing. It has also found utility in metallurgy as a desulfurizing agent and has historical applications in photography. These diverse uses underscore the significance of strontium nitrate across multiple industries.
Strontium Nitrate Formula FAQs
What is the molecular formula of strontium nitrate?
What are the physical properties of strontium nitrate?
What are the chemical properties of strontium nitrate?
What are some common applications of strontium nitrate?










