
As a coating and adhesive, urethane Formula is a carbamate-linked organic polymer chain that is derived from polymerizing ethanol and carbamic acid. It is a white powder or colourless crystal.
It is a polymer formed by condensation polymerisation of urethane, and is one of the most important urethane compounds. Aside from electrical potting, polyurethane is used to make fibres like spandex and PUL nut foams, accounting for 67% of the total usage. As a result of its insulation properties, it has a wide range of applications.
When heat causes decomposition, urethane Formula emits lethal nitrogen oxides. It is a colourless, odourless crystalline chemical that emits nitrogen oxides. In addition to producing amino resins, urethane can also be used to manufacture pesticides, fumigants, cosmetics, and medications. In general, urethane is a carcinogen that can harm the central nervous system, liver, and bone marrow.
Structure of Urethane
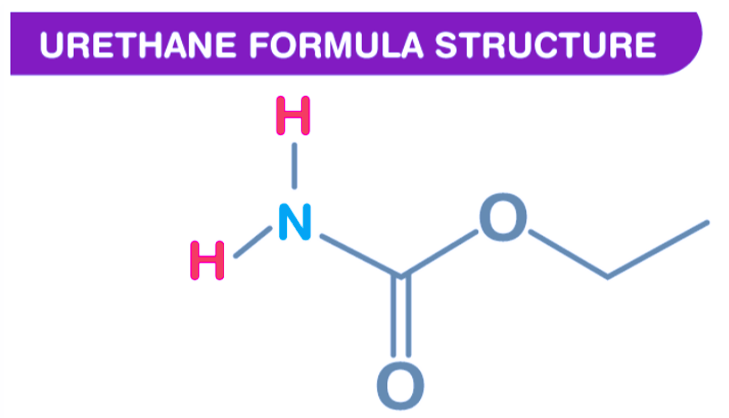
A molecule's structure refers to its arrangement of atoms and the chemical bonds that hold them together in chemistry. In urethane, there are 12 bonds in its molecule, of which 5 are non-H bonds, 1 multiple bond, 2 rotatable bonds, 1 double bond, and 1 (thio-) carbamate bond.
Physical Properties of Urethane
- As a white powder or crystals, Urethane Formula appears initially odourless and colourless.
- At 217°F and 54mmHg, it easily sublimates.
- The second type is colourless columnar crystals or white granular powder with a saline flavour.
- There is a bitter, cold, and saline taste to it.
- At 760 mm Hg, its boiling point ranges from 360 to 363 °F.
- The melting point of this metal is between 118 and 122 degrees Fahrenheit.
- 198 degrees Fahrenheit is its flashpoint.
- Lastly, it has a density of 0.9862 at 70 degrees F and a 100 mg/mL solubility at 72° F.
Chemical Properties of Urethane
Ethyl alcohol reacts with Urethane Formula to form polyurethane and water.
The combustion of urethane produces ammonia, carbon dioxide, and water.
Also Check – Atomic Mass Formula
Use of Urethane
Below are some of the uses of the Urethane Formula:
- In refrigerators, it is a key component of rigid and static foams.
- Foam makes car seats, bumpers, and doors more comfortable.
- Composite wood is attached to organic matter with polyurethane binders.
- Electronics industries use it to seal fragile, pressure-sensitive underwater cables.
Also Check – Tungstic Acid Formula
Safety Measures
Below are safety measures and health hazards:
- Exposure to this compound can harm the central nervous system and liver.
- In addition to causing bone marrow suppression, it may also cause human cancer.
- The decomposition of ethyl urethane releases toxic nitrogen oxide fumes.
Also Check – Bond Order Formula
Where Do We Use Urethane in Daily Life
Urethane is one of the most versatile materials which we could find in our daily lives. It is present in our machinery and other household items. Compared to synthetic material, we get to find out that it has several advantages, the latter one. This element is so flexible that it is used in comfort and relaxation products. In addition to this, that big machinery is made out of urethane as well. The other name of urethane is also called Polyurethane, which can be folded into foam that can be used in various products such as furniture, bedding, and seating.
On the other hand, it is used in insulation applications in many areas. As a result, you can find it in construction of the building as it also helps in insulating the walls while keeping the walls low density due to Polyurethane's rigidity. The urethane also helps keep the building warm during winter and cool during hot summer days. Moreover, when we compare it to other materials, it does have a long life span which is well over five decades, and it requires absolutely no maintenance. Footwear, especially the ones which are athletic-centric as urethane, provides both comfort and durability. You can find it in the midsole, and due to its elastic properties, it can be easily bent and stretched through the foot's natural motion. After daily usage, the urethane allows the footwear to get back in the older shape. Lastly, urethane naturally protects shoes and footwear from foul odor.
Urethane Formula FAQs
Q1. What is urethane in chemistry?
Q2. How is urethane commonly used in industry?
Q3. Is urethane toxic or hazardous to human health?
Q4. Can urethane be found in consumer products?
Q5. What are some environmental considerations regarding urethane products?










