
Zinc chloride formula is a fascinating chemical compound with a range of applications, and understanding its formula and properties is essential for both students and chemistry enthusiasts. In this article, we will explore zinc chloride's formula, structure, nomenclature, its charge, whether it's ionic or covalent, and a unique experiment involving its electrolysis.
Zinc Chloride Formula Structure
Zinc chloride exists as a white crystalline solid at room temperature. Its solid state forms a lattice structure where zinc cations (Zn²⁺) are surrounded by chloride anions (Cl⁻) in a three-dimensional pattern. This arrangement is held together by ionic bonds, which are strong electrostatic forces of attraction between oppositely charged ions. The chemical name for the compound with the formula ZnCl₂ is Zinc Chloride. This name reflects the combination of the metal zinc (Zn) and the chloride ion (Cl⁻).
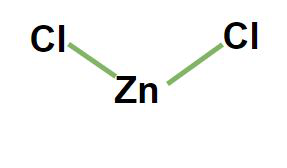
Zinc Chloride Formula by Criss Cross Method
The formula for zinc chloride is derived using the criss-cross method, which involves the exchange of charges between the cation (zinc) and anion (chloride). Zinc is a metal with a +2 charge, and chloride is a halogen with a -1 charge. When these two ions combine, the formula becomes ZnCl₂.
Zinc Chloride Formula Experiment
One interesting experiment involving zinc chloride is the synthesis of zinc chloride from its constituent elements - zinc (Zn) and chlorine (Cl₂). This experiment typically entails heating zinc in the presence of chlorine gas to produce zinc chloride. The balanced chemical equation for this reaction is:
Zn + Cl₂ → ZnCl₂
This experiment demonstrates the formation of an ionic compound through a chemical reaction.
Zinc Chloride Formula Charge
Zinc chloride's charge results from combining the charges of its constituent ions. As a metal, zinc forms Zn²⁺ cations, which means it has a charge of +2. On the other hand, chloride is a halogen that forms Cl⁻ anions with a charge of -1. When one zinc cation combines with two chloride anions, it produces a neutral compound, ZnCl₂.
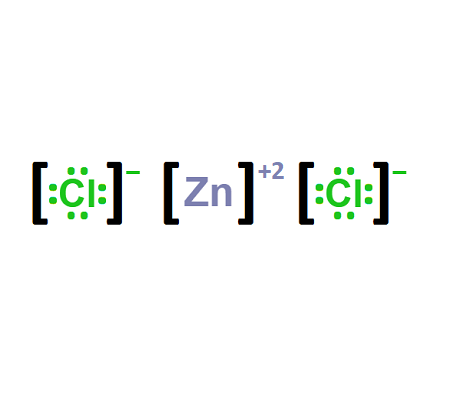
Zinc Chloride Formula: Ionic or Covalent?
Zinc chloride is an ionic compound. Ionic compounds are formed by transferring electrons from one element to another, resulting in the attraction between positively and negatively charged ions. In the case of zinc chloride, the metal zinc donates two electrons to chlorine, forming an ionic bond between Zn²⁺ and Cl⁻ ions.
Zinc Chloride Formula Electrolysis
Electrolysis is when an electric current is passed through an electrolyte (a substance that can conduct electricity when dissolved or melted) to induce a chemical reaction. In the case of zinc chloride, it can be electrolyzed to obtain zinc metal and chlorine gas. This process is used in the purification of zinc.
The electrolysis of zinc chloride involves passing an electric current through a molten or aqueous solution of ZnCl₂. At the anode, chloride ions (Cl⁻) are oxidized to release chlorine gas (Cl₂), while at the cathode, zinc ions (Zn²⁺) are reduced to form zinc metal (Zn). This experiment demonstrates the versatility of zinc chloride in various chemical processes.
Preparation of Zinc Chloride
The reaction between metallic zinc and hydrogen chloride gas forms anhydrous zinc chloride. The chemical equation for this reaction is as follows:
Zn + 2HCl → ZnCl 2 + H 2
Hydrochloric acid can be used to treat zinc as an alternative to hydrogen chloride. Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide.
ZnS + 2HCl → ZnCl 2 + H 2 S
The only oxidation state of zinc is +2, so its purification is relatively straightforward.
Properties Of Zinc Chloride
Zinc chloride is a remarkable chemical compound with a wide array of properties that make it invaluable in various industrial, scientific, and practical applications. From its physical characteristics to its chemical behavior, understanding the properties of zinc chloride is crucial for harnessing its potential.
| Zinc Chloride Properties | |
| Name | Zinc Chloride |
| Also Known as | Zinc Butter, Zinc dichloride |
| Appearance | Colourless or white crystals |
| Molecular Formula | ZnCl 2 |
| Melting Point of Zinc Chloride | 275 °C |
| Boiling Point of Zinc Chloride | 756 °C |
| Density | 2.91 g/cm³ |
| Molar Mass | 136.286 g/mol |
| Solubility in Water | Soluble in water |
Zinc Chloride Uses
In its molten state, this compound catalyzes various aromatization reactions, including the synthesis of hexamethylbenzene from methanol. Additionally, acting as a moderately strong Lewis acid, it facilitates Fischer indole synthesis and certain Friedel-Crafts acylation reactions. This versatile reagent is also highly effective in the production of alkyl chlorides. For instance, it is commonly used in creating smoke bombs, where it reacts with hexachloroethane to produce Zinc Chloride Formula smoke that acts as a smoke screen upon ignition.
Furthermore, ZnCl2 has practical applications in fingerprint recognition due to its ability to form a distinguishable complex with Luhemann violet. When diluted, aqueous solutions of this compound can be utilized for disinfection purposes. It is even found as an ingredient in some antiseptic mouthwash formulas.
| Related Links | |
| Zinc Iodide formula | Zinc Sulfide Formula |
| aluminium carbonate formula | Bromine Formula |
Zinc Chloride Formula FAQs
What is the formula of zinc chloride?
What is the correct name for ZnCl₂?
Why is there a 2 in zinc chloride?
What is the formula for zinc chloride with Valency?










