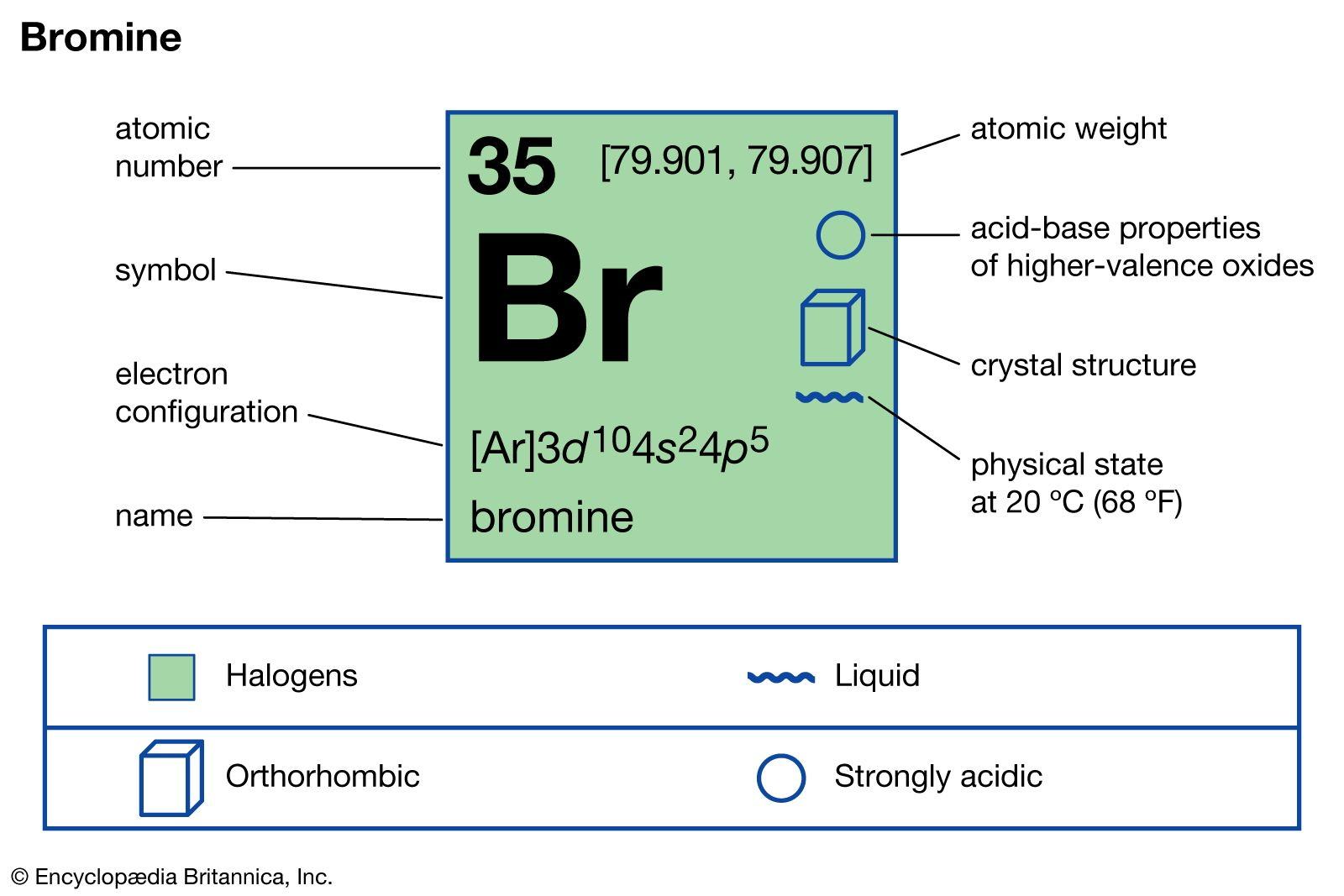
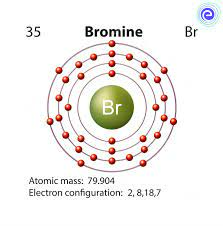
Bromine formula another term is Dibromine or Brome. Classified as a halogen with the atomic symbol Br, it was first discovered in 1825-1826 by Antoine Ballard and Carl Jacob Lowing. Brome, a volatile liquid with a reddish-brown hue, has a pungent odor and emits suffocating vapors. It is both soluble in water and denser than water. Additionally, it possesses toxic and corrosive properties. Bromine is a fascinating element with the chemical symbol "Br" and atomic number 35. It is a non-metallic halogen found in the periodic table, and it possesses several intriguing aspects that make it an essential subject of study in chemistry. Let's explore the various aspects of bromine's formula and its properties.
Bromine Formula and Valency
The bromine formula is quite simple – it is represented as "Br." Bromine has a valency of 1, which means it can form a single bond with another atom or molecule. This property allows bromine to engage in various chemical reactions, making it an important component in the world of organic chemistry.
Also Read : Ammonium Sulfide Formula
Bromine Formula Structure
Bromine formula exists as diatomic molecules in its natural state. Two bromine atoms share a covalent bond, forming Br2. This diatomic molecule has a reddish-brown color and is highly volatile. Its molecular formula, Br2, showcases its distinctive structure, where the two bromine atoms share a single covalent bond.
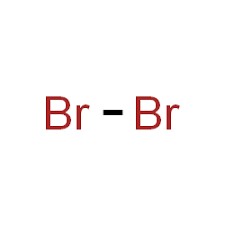
Also Read : Ammonium Dichromate Formula
Bromine Formula in Standard State
In its standard state, bromine is a diatomic molecule, as mentioned earlier. It is a liquid at room temperature, with a boiling point of 58.8 degrees Celsius (137.8 degrees Fahrenheit). At this temperature and pressure, the formula for bromine remains Br2.
Bromine Formula and Charge
Bromine formula has a unique charge configuration when it forms ions. It can either gain one electron to become a bromide ion (Br-) or share electrons with other atoms in covalent compounds. This ability to change its charge state makes bromine a versatile element in chemical reactions.
Bromine Formula Mass
The molar mass of bromine, which is the mass of one mole of bromine atoms or molecules, is approximately 79.904 grams per mole. This mass is crucial for calculating the amounts of bromine involved in chemical reactions and for understanding its properties in various contexts.
Bromide Ion Formula
When bromine gains an electron, it becomes a bromide ion with the formula Br-. Bromide ions are negatively charged and often found in ionic compounds. They play a significant role in various chemical processes, particularly in the formation of salts.
Bromine Formula Gas
Bromine is a volatile element, and when heated, it can transition from its liquid state to a gaseous state. As a gas, it can be toxic, and its fumes are highly irritating. Handling bromine gas requires appropriate safety measures due to its hazardous nature.
Bromine Formula Liquid
At room temperature, bromine exists in its liquid state. It is the only non-metallic element that is naturally liquid under normal conditions. Its liquid form, Br2, is known for its vivid reddish-brown color and high volatility.
Bromine can combine with various elements to form a wide range of compounds. In these compounds, bromine's formula varies based on its oxidation state and the atoms it bonds with. These compounds can have diverse properties and applications in industries like pharmaceuticals, agriculture, and materials science.
Also Read : Argon Gas formula
Physical and Chemical Properties of Bromine
- A reddish brown liquid with a noticeable vapour pressure, free bromine is at room temperature. Despite being highly irritating to the skin, eyes, and respiratory system, bromine vapour has a strong odour and can be lethal if exposed for a short period of time. As with other halogens, bromine exists as diatomic molecules in all aggregation states.
- At room temperature, a 3.41-gram (0.12-ounce) amount of bromine easily dissolves in 100 millilitres (0.1 quart) of water, forming what is commonly known as bromine water. Acting as an effective oxidising agent similar to chlorine water, it has the added advantage of being more stable and less prone to breakdown. Bromine water can extract free iodine and sulphur from liquids that contain iodide and hydrogen sulphide respectively. Additionally, it has the ability to oxidise sulphurous acid into sulfuric acid. When exposed to sunlight, bromine water decomposes and releases oxygen, as illustrated by the following equation:
- The bromide ion is less hydrated than the chloride ion, so it is a less effective oxidizing agent. Similarly, metal-bromine bonds are weaker than metal-chlorine bonds, which is evident in bromine's chemical reactivity, which is intermediate between chlorine and iodine. It is common for organic bromo compounds to be denser, less volatile, less flammable, and less stable than their chloro derivative counterparts.
- While bromine reacts violently with alkali metals, phosphorus, arsenic, aluminum, and antimony, it reacts less violently with other metals. Bromine adds to unsaturated hydrocarbons and displaces hydrogen from saturated hydrocarbons, but not as easily as chlorine.
- Bromine exhibits various oxidation states, including its most stable state of +1, which is naturally occurring. Other identified states include 0 (elemental bromine, Br2), +1 (hypobromite, BrO), +3 (bromite, BrO2), +5 (bromate, BrO3), and +7 (perbromate, BrO4). Its high initial ionisation energy makes it difficult to form compounds with positive oxidation states. However, suitable ligands such as oxygen and fluorine can stabilize these states. Covalent bonds are commonly found in compounds with oxidation numbers ranging from +1 to +7.
Uses of Bromine
- Silver bromide, which is used alone or in combination with silver chloride and silver iodide, is the light-sensitive element in photographic emulsions.
- In gasoline containing lead, ethylene bromide was used as an anti-knocking agent.
- As a pesticide, poisonous bromomethane was often used to fumigate soil and houses
- In the 19th and early 20th centuries, inorganic bromide compounds, particularly potassium bromide, were widely used as sedatives.
- Organobromine compounds have a variety of applications, including high-density drilling fluids, dyes like Tyrian purple and bromothymol blue, and medications. They are also used in water treatment and as the starting material for many inorganic chemicals. For example, silver bromide is often used in photography. On a larger scale, zinc-bromine batteries are hybrid flow batteries that provide stationary electrical power backup and storage for both residential and industrial purposes.
| Related Links | |
| Carbonous Acid Formula | Cholestrol Formula |
| Chlorine Gas Formula | Chromic Acid Formula |
Bromine Formula FAQs
Is bromine a Br2 or Br?
What is bromine in formula?
What is the symbol of bromine?
How is bromine named?