
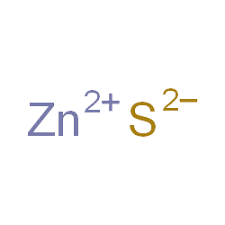
A Zinc Sulfide formula, also known as Zincblende or Wurtzite. A zinc metal-sulfur atom is attached to each other via a polar covalent bond in Zincblende. Zinc Sulfide has the chemical or molecular formula ZnS.
The crystal form of this substance can vary in color, ranging from white to yellowish-white. It is typically found as a greyish white mineral known as sphalerite, or zinc blende. ZnS has a higher density than water and is not soluble in it. It can be created through the combustion of sulfur and zinc, or by reacting zinc sulfate with sodium sulfide, or by introducing hydrogen sulfide gas into a solution containing Zn2+. When formed, it displays luminescent properties that make it useful in various industries.
There is no harm to humans from ZnS, but it can be hazardous to the environment. It can penetrate the soil and contaminate the groundwater, so immediate action needs to be taken.
Zinc Sulfide Formula
Zinc sulfide is a chemical compound with the formula ZnS. It is composed of zinc (Zn) cations and sulfide (S2-) anions. This formula represents the basic building blocks of the compound and provides important information about its chemical composition. The systematic name for ZnS is zinc sulfide, as per the IUPAC (International Union of Pure and Applied Chemistry) nomenclature. This name reflects the composition of the compound and follows the standard naming conventions in chemistry.Also Read: Ammonium Iodide Formula
Zinc Sulfide Formula Structure
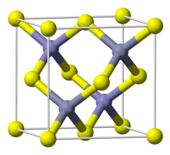
The mineral zinc sulphide, also known as ZnS, exists in two main crystalline forms. This illustrates a phenomenon known as polymorphism. Both forms have a tetrahedral coordination geometry at the Zn and S elements. Additionally, they are referred to as zinc blende or sphalerite for the cubic form and wurtzite for the hexagonal form. The hexagonal form can be produced synthetically and occurs when the temperature reaches approximately 1020 °C, causing a shift from sphalerite to wurtzite. There is also a rare tetragonal form called Polhemus site with the formula (Zn, Hg)S.
Zinc Sulfide Formula Charge
In the formula ZnS, the zinc cation (Zn) has a charge of +2, and the sulfide anion (S2-) carries a charge of -2. These charges balance each other out, resulting in a neutral compound. The balanced charges are a key characteristic of ionic compounds like zinc sulfide.
Zinc Sulfide Formula Balanced Equation
A balanced chemical equation can represent the formation of zinc sulfide. When zinc reacts with sulfur, it forms zinc sulfide, and the equation can be written as follows: Zn + S → ZnS This equation shows that one zinc atom combines with one sulfur atom to produce one zinc sulfide molecule.Zinc Sulfide Formula Solid
Zinc sulfide exists in various forms, including both crystalline and amorphous structures. The crystalline form of zinc sulfide is a white-to-yellowish solid with applications in luminescent materials, pigments, and phosphors. It is often used in producing glow-in-the-dark products due to its ability to emit light when exposed to ultraviolet (UV) or other forms of radiation.Zinc Sulfide Formula in Chemistry
In chemistry, zinc sulfide is a widely studied compound with various applications. Its luminescent properties make it valuable in the manufacturing of phosphors used in television screens, fluorescent lamps, and X-ray screens. It is also utilized as a pigment in the creation of white and yellow paints. In addition, it plays a role in the field of semiconductors, where it is used in the production of optoelectronic devices like photodetectors.Zinc Sulfide Formula by Criss Cross Method
The criss-cross method is a simple way to determine the formula of an ionic compound. In the case of zinc sulfide (ZnS), you can determine the formula by swapping the charges of the cation and anion:- Zinc (Zn) has a charge of +2.
- Sulfide (S2-) has a charge of -2.
Zinc Sulfide Properties
| Chemical formula | ZnS |
| Molecular weight | 97.474 g/mol |
| Density | 4.090 g/mL |
| Boiling point | 1,935 °C |
| Melting point | 1,850 °C |
| Related Links | |
| Ammonium Dichromate Formula | Aluminium Iodide Formula |
| Ammonium Iodide Formula | Water formula |
Zinc Sulfide Formula FAQs
How do you write Zinc Sulfide?
Zinc sulfide is typically written as ZnS in its chemical formula.
What is ZnS chemical name?
The chemical name for ZnS is zinc sulfide.
Is calamine a ZnS?
Yes, calamine is a mineral that primarily consists of zinc carbonate (ZnCO3) and a mixture of other compounds. While it contains zinc, it is not the same as zinc sulfide (ZnS).
What is the full form of zinc sulphide?
The full form of zinc sulphide is the same as the chemical name, which is "zinc sulfide." It is commonly spelled as "sulfide" in American English and "sulphide" in British English.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









